The Molecules of Cells Part I Introduction Carbohydrates
The Molecules of Cells Part I – Introduction; Carbohydrates; Lipids
Organic Chemistry Study of carbon compounds Occur in more than just living things Are typically made by living things – Organic compounds come from organisms – The chemistry that you study in HS is inorganic Compounds that are found in the non-living world http: //www. chemistryland. com/Elementary. School/Building. Blocks/Building. Organic. htm 2
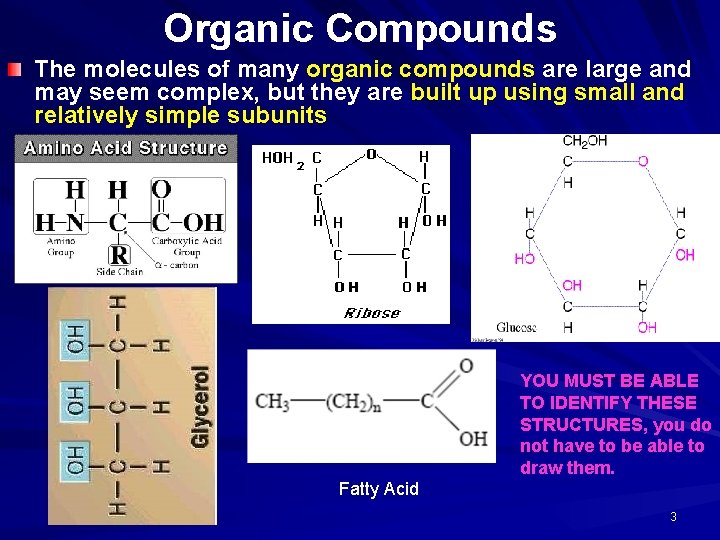
Organic Compounds The molecules of many organic compounds are large and may seem complex, but they are built up using small and relatively simple subunits YOU MUST BE ABLE TO IDENTIFY THESE STRUCTURES, you do not have to be able to draw them. Fatty Acid 3

Organic Compounds containing carbon that occur in living organisms are regarded as organic. –Exceptions: carbonates and oxides of carbon (e. g. CO 2)

A carbon atom has 4 valence electrons Each valence electron can join with an electron from another atom to form a strong covalent bond. Therefore, one carbon can form bonds with up to four other atoms.
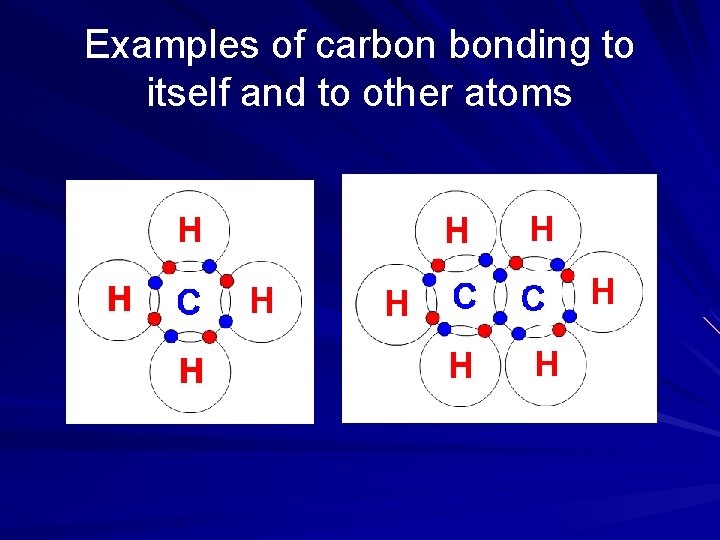
Examples of carbon bonding to itself and to other atoms
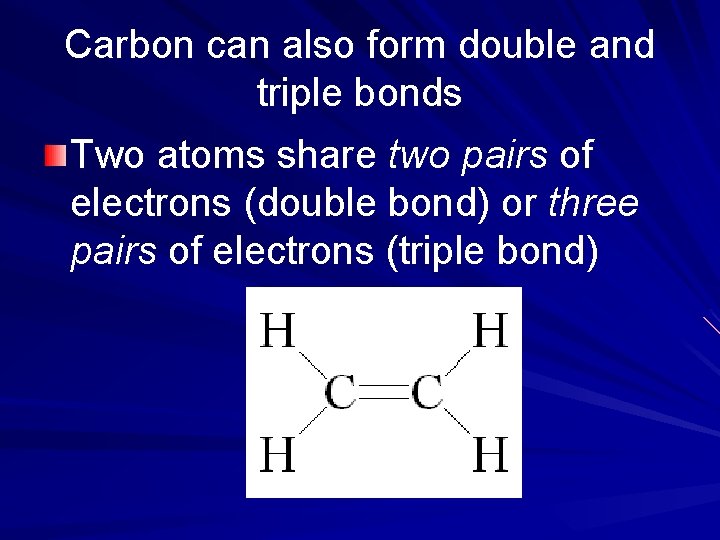
Carbon can also form double and triple bonds Two atoms share two pairs of electrons (double bond) or three pairs of electrons (triple bond)
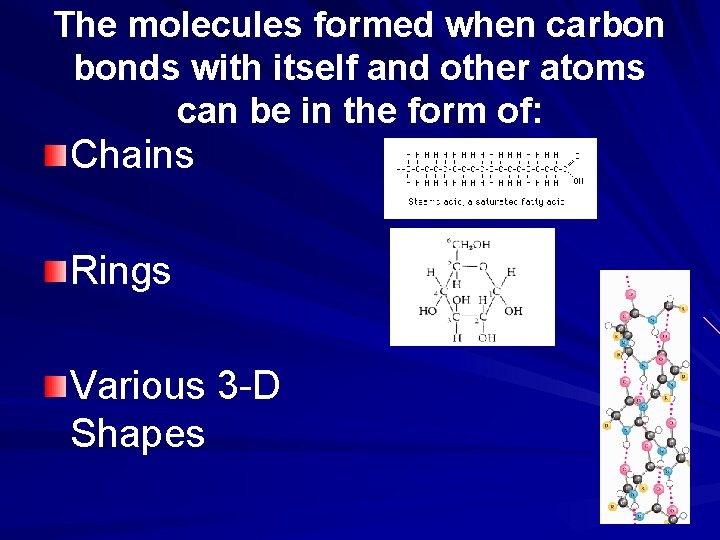
The molecules formed when carbon bonds with itself and other atoms can be in the form of: Chains Rings Various 3 -D Shapes
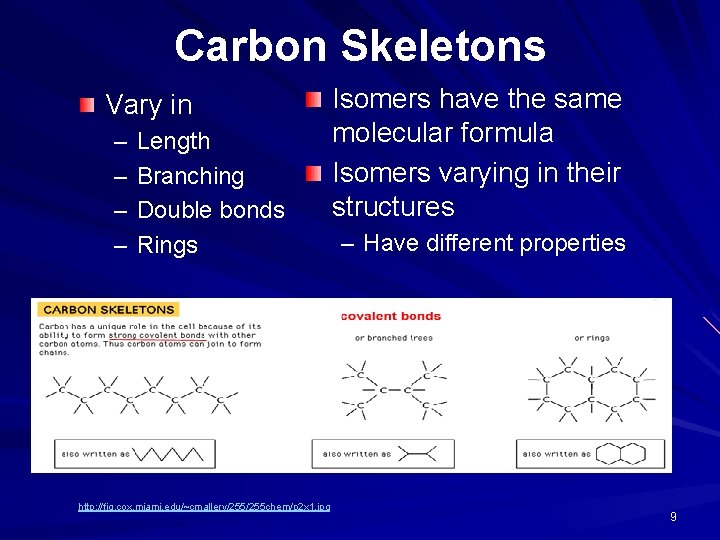
Carbon Skeletons Vary in – – Length Branching Double bonds Rings http: //fig. cox. miami. edu/~cmallery/255 chem/p 2 x 1. jpg Isomers have the same molecular formula Isomers varying in their structures – Have different properties 9
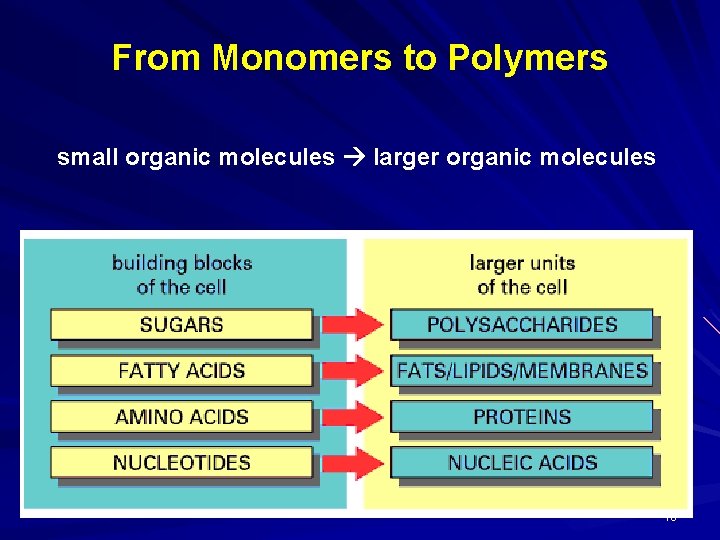
From Monomers to Polymers small organic molecules larger organic molecules 10
Molecular Diversity and Complexity of Living Organisms Results from: variation in types of carbon skeletons that are possible diversity of atoms of other elements that can be bonded to the skeletons at available sites
Important Concepts Almost the entire dry weight of living organisms is composed of extremely large organic molecules (organic macromolecules). Organic macromolecules are synthesized from simple subunits. Organic macromolecules have many diverse structures and functions.
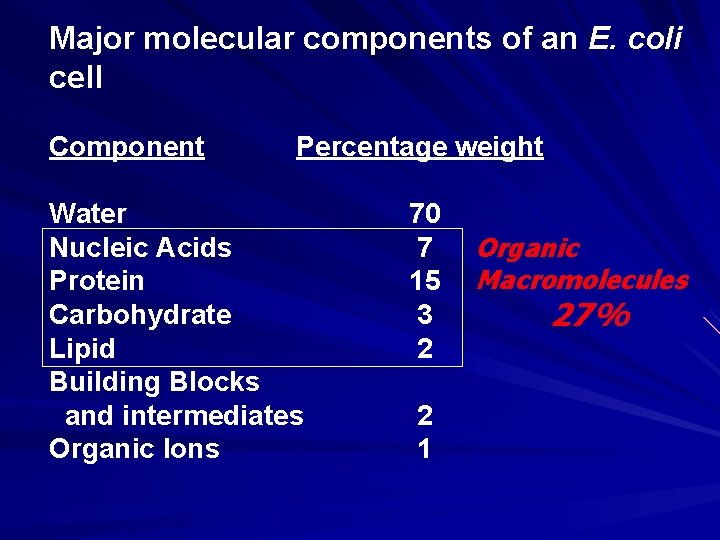
Major molecular components of an E. coli cell Component Percentage weight Water Nucleic Acids Protein Carbohydrate Lipid Building Blocks and intermediates Organic Ions 70 7 15 3 2 2 1 Organic Macromolecules 27%

Macromolecules Synthesized from smaller subunits or building blocks – building block = monomer – macromolecule = polymer

Formation and Breakdown of Organic Macromolecules Condensation –Joins monomers to form polymers - water is removed Hydrolysis –Breaks down polymers to form monomers – water is added
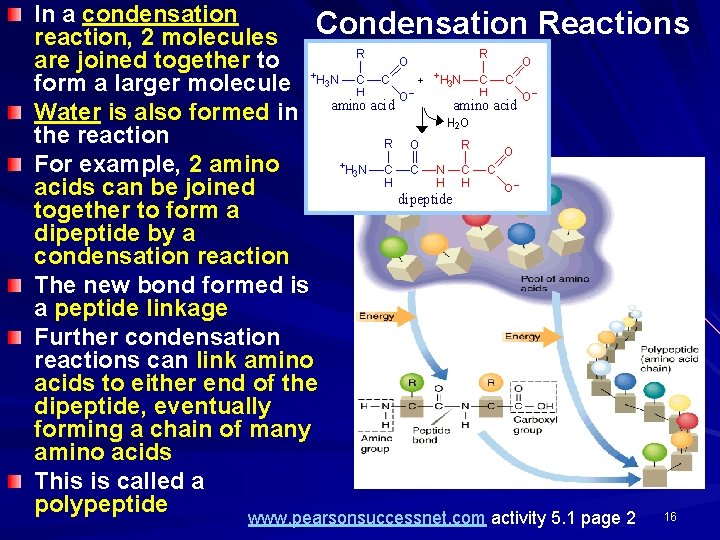
In a condensation Condensation Reactions reaction, 2 molecules are joined together to form a larger molecule Water is also formed in the reaction For example, 2 amino acids can be joined together to form a dipeptide by a condensation reaction The new bond formed is a peptide linkage Further condensation reactions can link amino acids to either end of the dipeptide, eventually forming a chain of many amino acids This is called a polypeptide 16 www. pearsonsuccessnet. com activity 5. 1 page 2
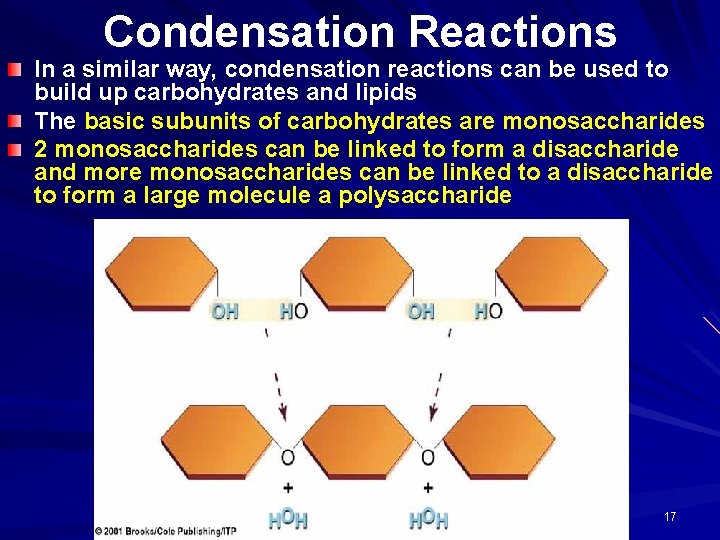
Condensation Reactions In a similar way, condensation reactions can be used to build up carbohydrates and lipids The basic subunits of carbohydrates are monosaccharides 2 monosaccharides can be linked to form a disaccharide and more monosaccharides can be linked to a disaccharide to form a large molecule a polysaccharide 17
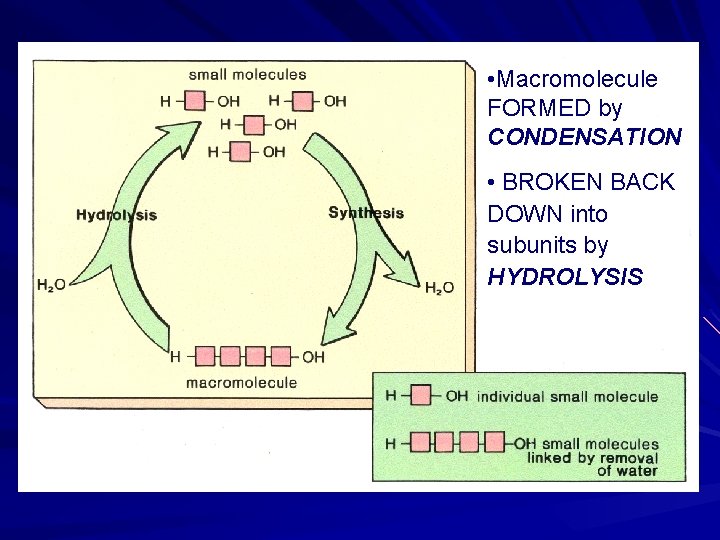
• Macromolecule FORMED by CONDENSATION • BROKEN BACK DOWN into subunits by HYDROLYSIS
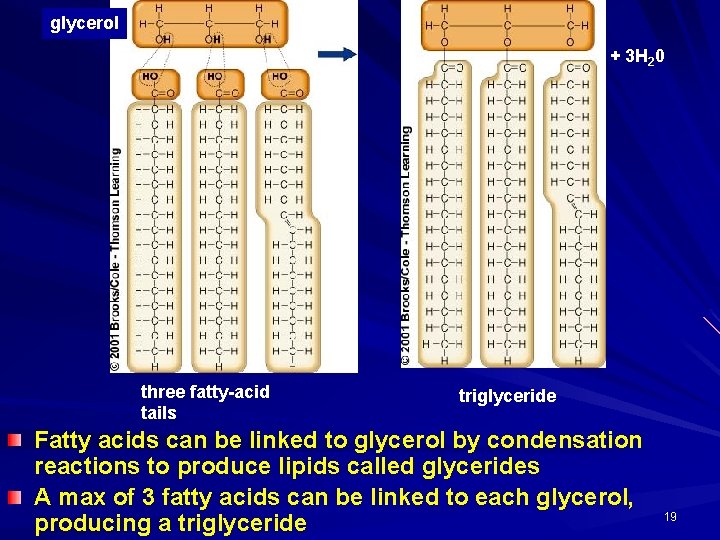
glycerol + 3 H 20 three fatty-acid tails triglyceride Fatty acids can be linked to glycerol by condensation reactions to produce lipids called glycerides A max of 3 fatty acids can be linked to each glycerol, producing a triglyceride 19
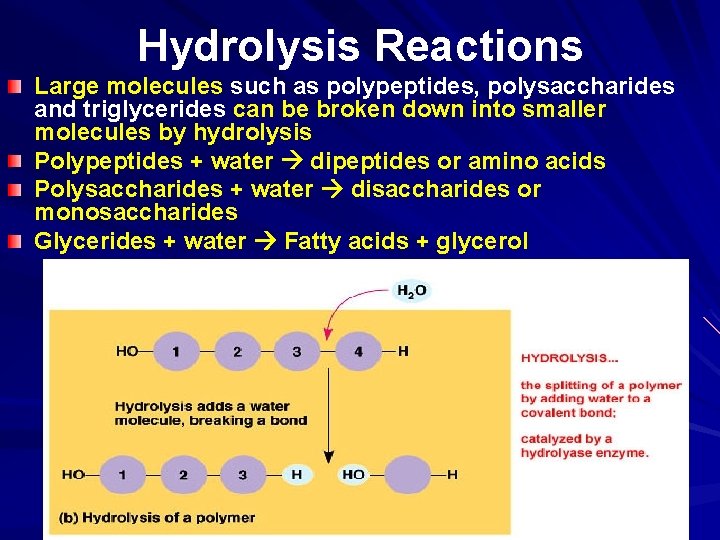
Hydrolysis Reactions Large molecules such as polypeptides, polysaccharides and triglycerides can be broken down into smaller molecules by hydrolysis Polypeptides + water dipeptides or amino acids Polysaccharides + water disaccharides or monosaccharides Glycerides + water Fatty acids + glycerol 20
Classes of Organic Macromolecules in Cells Carbohydrates Lipids Proteins Nucleic Acids
Carbohydrates Building blocks (monomers) are simple sugars called monosaccharides. Function in energy storage and cell structure
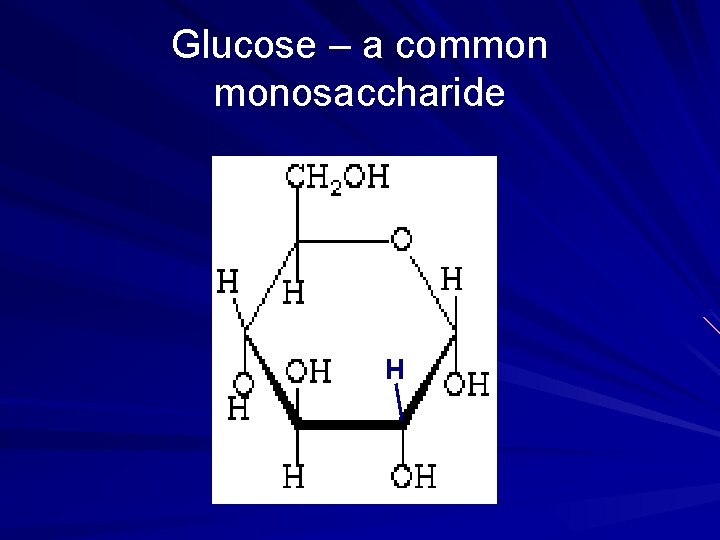
Glucose – a common monosaccharide H
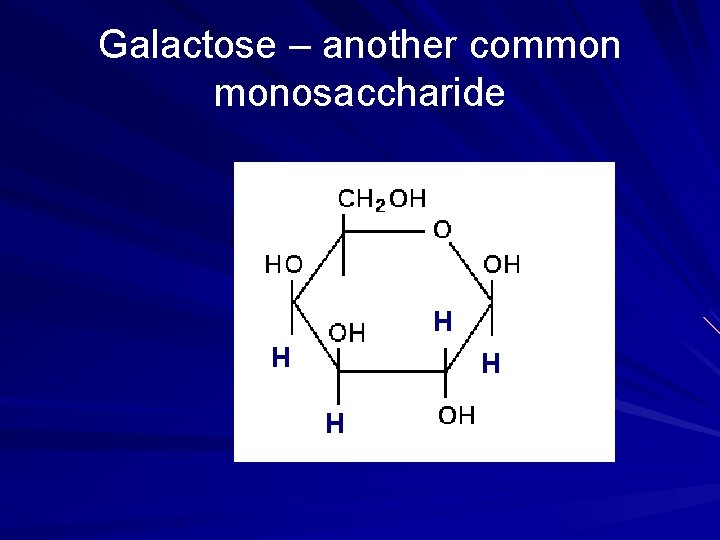
Galactose – another common monosaccharide H H
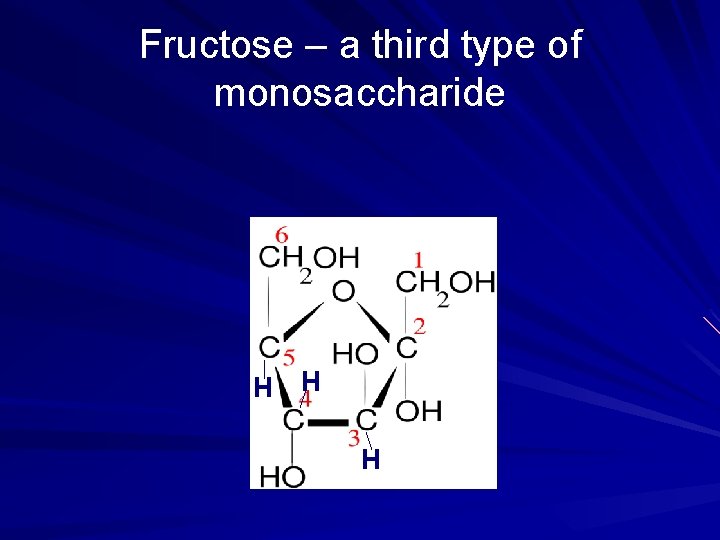
Fructose – a third type of monosaccharide H H H
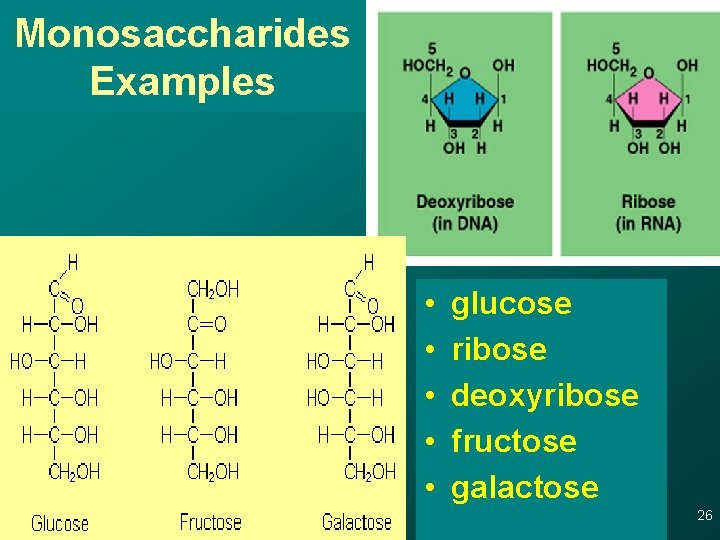
Monosaccharides Examples • • • glucose ribose deoxyribose fructose galactose 26
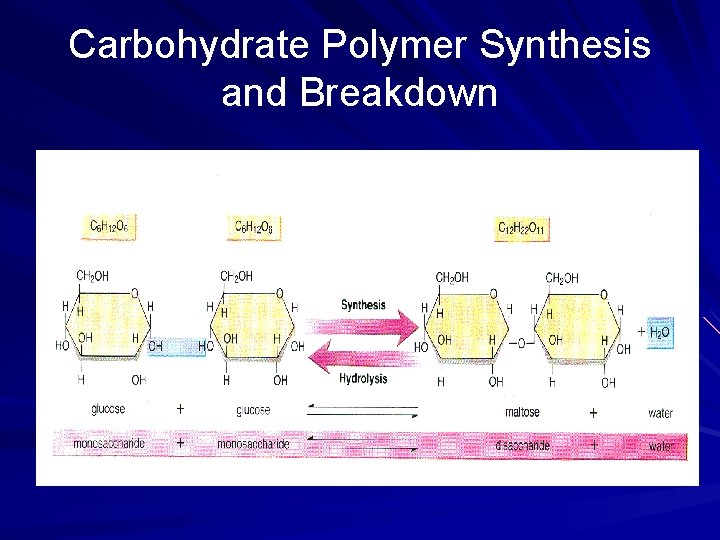
Carbohydrate Polymer Synthesis and Breakdown
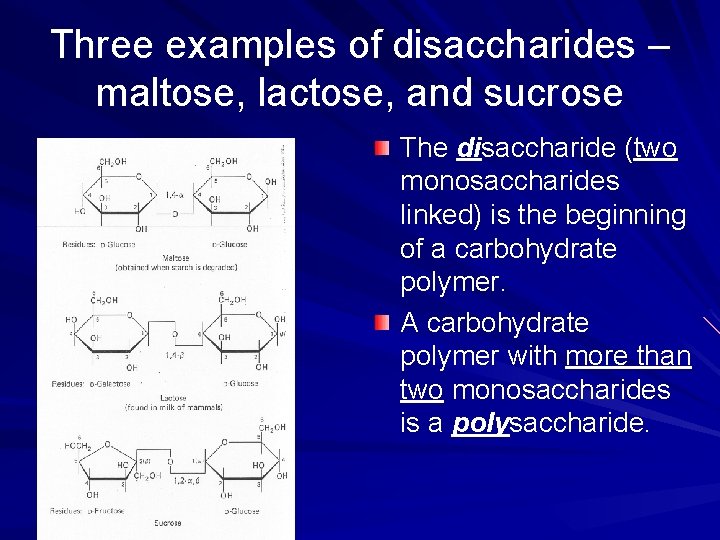
Three examples of disaccharides – maltose, lactose, and sucrose The disaccharide (two monosaccharides linked) is the beginning of a carbohydrate polymer. A carbohydrate polymer with more than two monosaccharides is a polysaccharide.
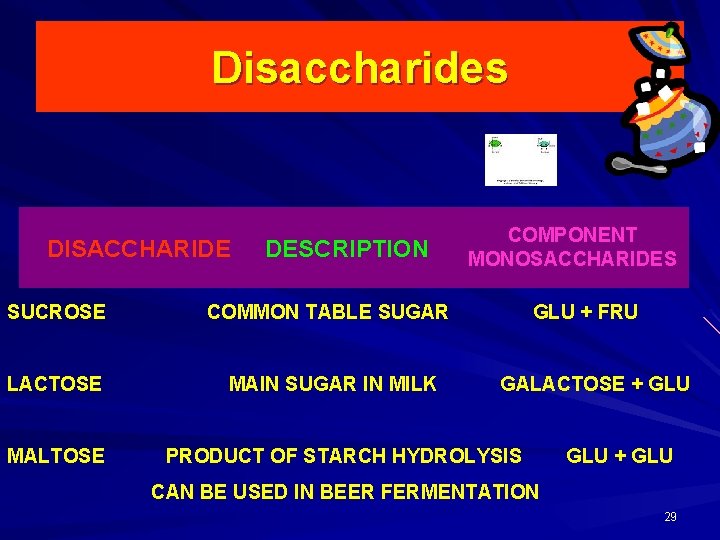
Disaccharides DISACCHARIDE DESCRIPTION SUCROSE COMMON TABLE SUGAR LACTOSE MAIN SUGAR IN MILK MALTOSE COMPONENT MONOSACCHARIDES GLU + FRU GALACTOSE + GLU PRODUCT OF STARCH HYDROLYSIS GLU + GLU CAN BE USED IN BEER FERMENTATION 29
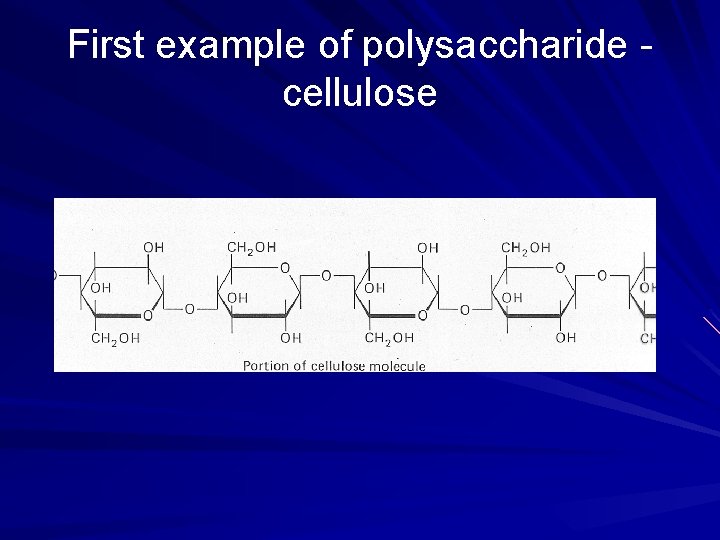
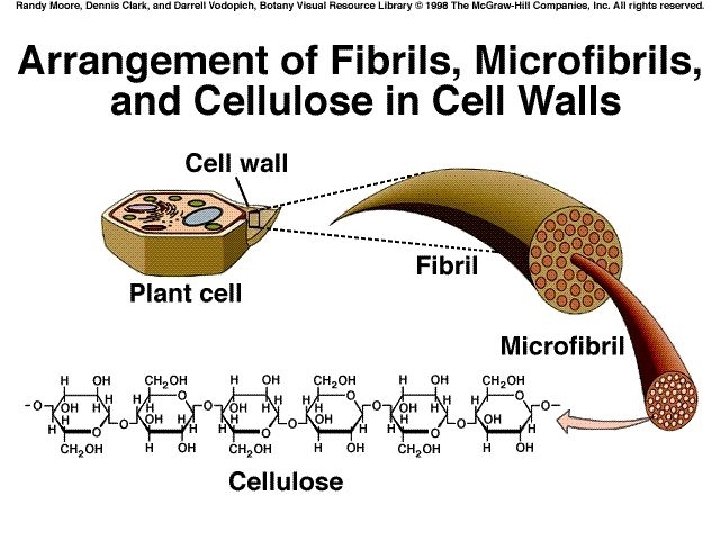
First example of polysaccharide cellulose
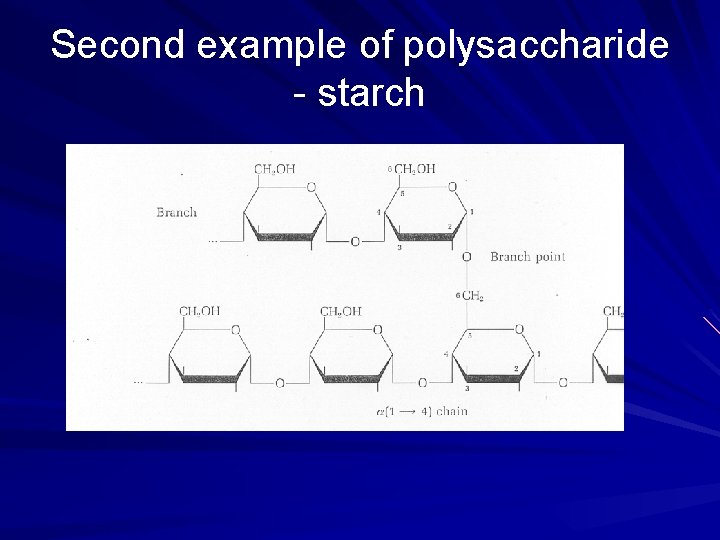
Second example of polysaccharide - starch
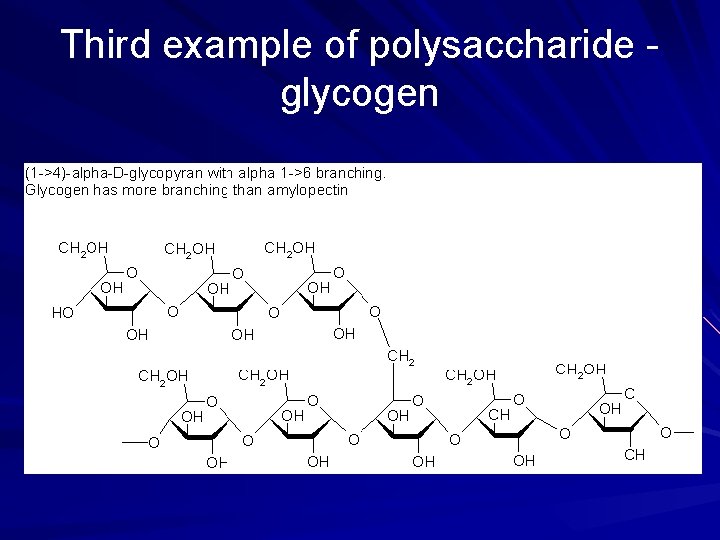
Third example of polysaccharide glycogen

Some functions of carbohydrates in animals Glucose: broken down in cellular respiration to release energy Lactose: the sugar in the milk produced by mammals Glycogen: energy store in liver and skeletal muscles

Some functions of carbohydrates in plants Fructose: energy source and component of sucrose Sucrose: unreactive, and so a good way to transport sugar throughout the plant Cellulose: main component of the cell wall
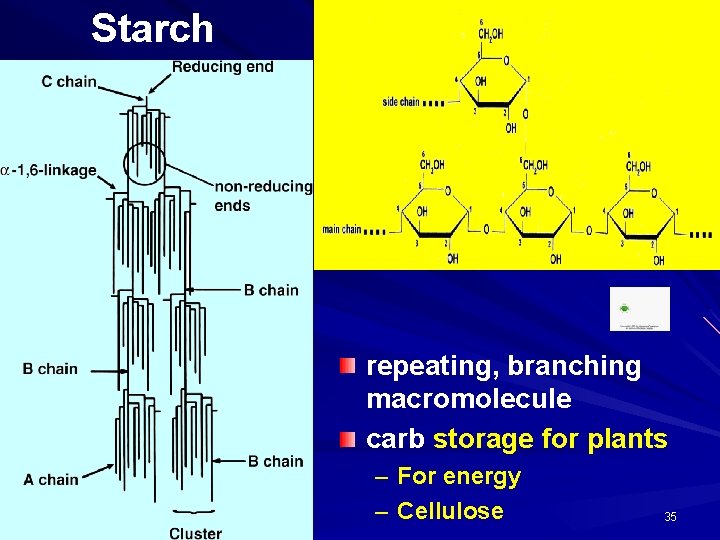
Starch repeating, branching macromolecule carb storage for plants – For energy – Cellulose 35
36
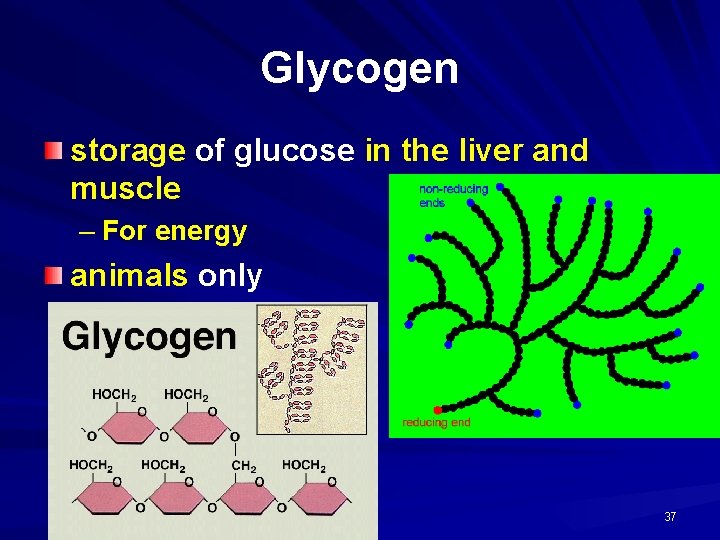
Glycogen storage of glucose in the liver and muscle – For energy animals only 37
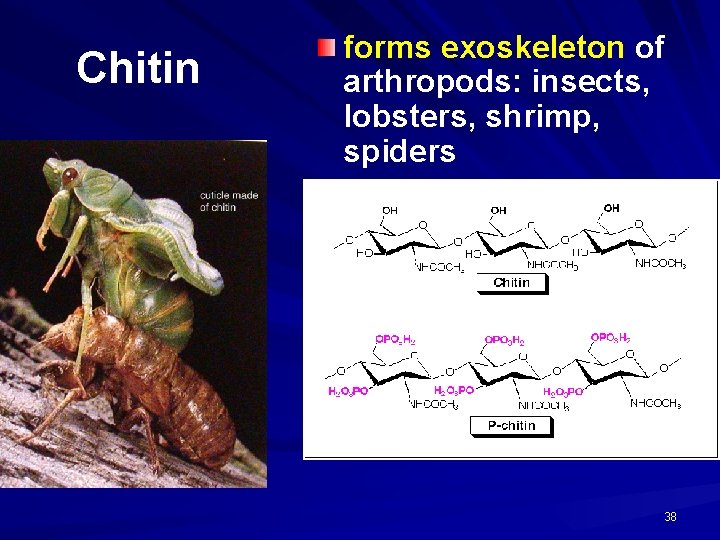
Chitin forms exoskeleton of arthropods: insects, lobsters, shrimp, spiders 38
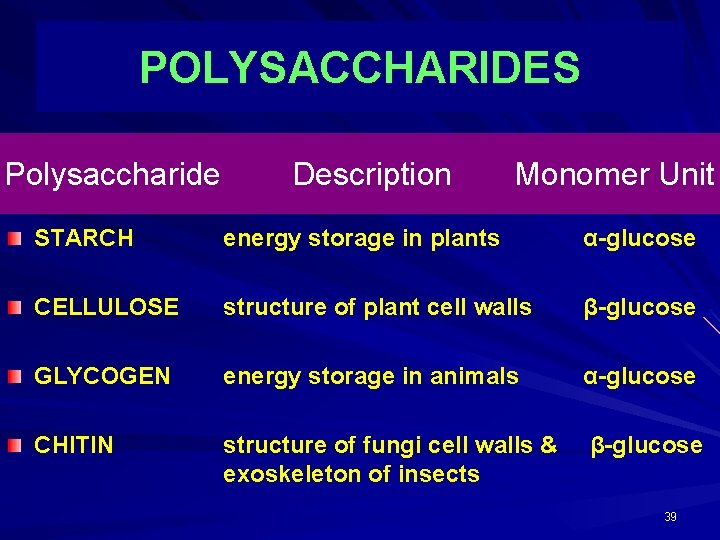
POLYSACCHARIDES Polysaccharide Description Monomer Unit STARCH energy storage in plants α-glucose CELLULOSE structure of plant cell walls β-glucose GLYCOGEN energy storage in animals α-glucose CHITIN structure of fungi cell walls & exoskeleton of insects β-glucose 39
- Slides: 39