The Molecules of Cells An Introduction to BIOCHEMISTRY
The Molecules of Cells An Introduction to BIOCHEMISTRY
2. 1 Basic chemistry l Matter refers to anything that has mass or volume o Exists in 3 states l All matter (living or non-living) is composed of elements l Elements consist of atoms l Atoms consist of protons, neutrons and electrons

CHNOPS? l There are only 6 elements that are basic to life and make up ~95% of the body weight of organisms l Other elements that are important to living things are Na, K, Ca, Fe, and Mg

The 3 subatomic particles Particle Location Charge Mass PROTONS Nucleus +1 1 amu NEUTRONS Nucleus 0 ~1 amu ELECTRONS Orbitals -1 ~0 amu
Atoms of the same element… l Have the same number of protons l The # of protons = atomic # l This is what makes an atom unique l # protons + # neutrons = atomic mass o Because e-s are so light, they don’t contribute to an atom’s mass! Example: o Carbon has 6 protons atomic mass = 12 amu . : has 6 neutrons l
Isotopes: l Atoms of the same element that differ in their number of neutrons o The identity of the atom doesn’t change, but the mass number does! l Example: Carbon-12 VS. Carbon-14 6 protons 6 neutrons 6 protons 8 neutrons
Isotopes… l Radioactive isotopes are unstable atoms that spontaneously emit radiation in the form of radioactive particles or radiant energy l Can be used as medical tracers, as they show up on PET scans l High levels of radiation can harm cells, damaging DNA, and cause cancer; but can be used to sterilize medical/dental equipment, etc.
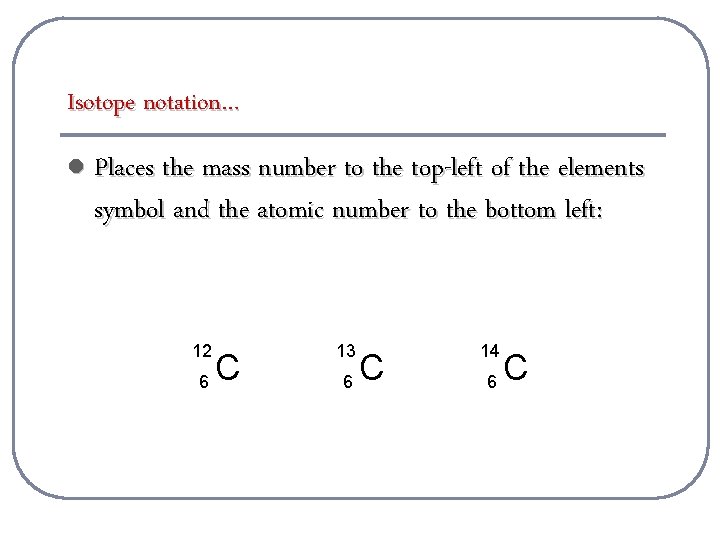
Isotope notation… l Places the mass number to the top-left of the elements symbol and the atomic number to the bottom left: 12 6 C 13 6 C 14 6 C

A bit about electrons… l The number of valence electrons in an atom determines whether and how that atom will react with others l Atoms with full valence shells will likely not react • l Ex. Noble gases Atoms with partially full valence shells will react with other atoms to achieve a full octet! • Ex. Oxygen, chlorine, sodium, etc…
The periodic table l Constructed to group the elements according to chemical and physical properties
2. 2 Molecules and compounds: l A molecule is formed when two or more atoms bond together l When the atoms of two or more different elements bond together, the product is called a compound
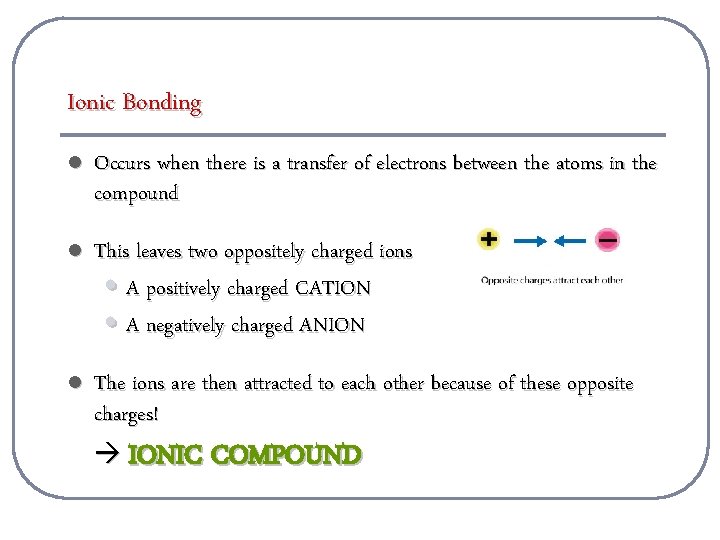
Ionic Bonding l Occurs when there is a transfer of electrons between the atoms in the compound l This leaves two oppositely charged ions • A positively charged CATION • A negatively charged ANION l The ions are then attracted to each other because of these opposite charges! IONIC COMPOUND
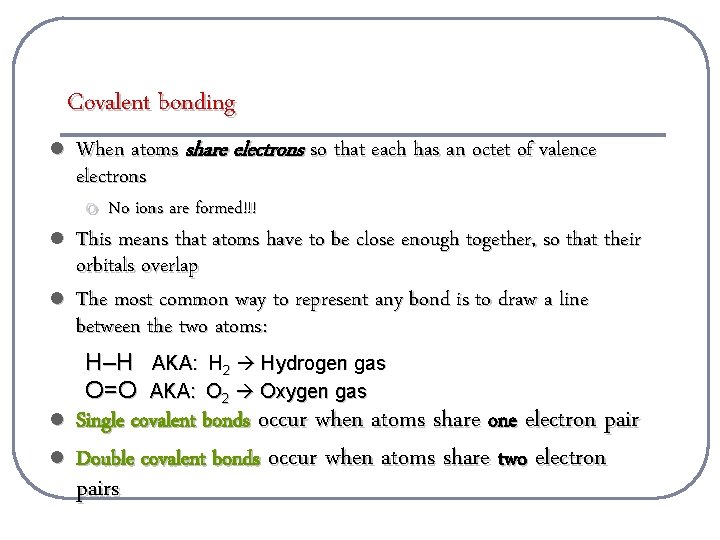
Covalent bonding l l l When atoms share electrons so that each has an octet of valence electrons o No ions are formed!!! This means that atoms have to be close enough together, so that their orbitals overlap The most common way to represent any bond is to draw a line between the two atoms: H–H AKA: H 2 Hydrogen gas O=O AKA: O 2 Oxygen gas l l Single covalent bonds occur when atoms share one electron pair Double covalent bonds occur when atoms share two electron pairs
Shapes of molecules l l Molecules are not one dimensional! Their 3 D shape is actually important in determining their biological function o Ex. Hormones have specific shapes that allow them to be recognized by the cells in the body o Antibodies also combine with pathogens, like a key fits a lock, to keep us well o And, HOMEOSTASIS is maintained only when enzymes have the proper shape to carry out their role in chemical rxns.

Polar and Non-polar covalent bonds l Unequal sharing of e-s = polar covalent bond o This creates partial charges on O and H atoms l Equal sharing of e-s = non-polar covalent bond l Some atoms are more electronegative than others o That is, they attract electrons to a greater extent o This means that in a bond, electrons spend more time orbiting one of the atoms o. : At any given time, one of the atoms has a slightly negative charge, while the o other, a slightly positive charge EXAMPLE: WATER!
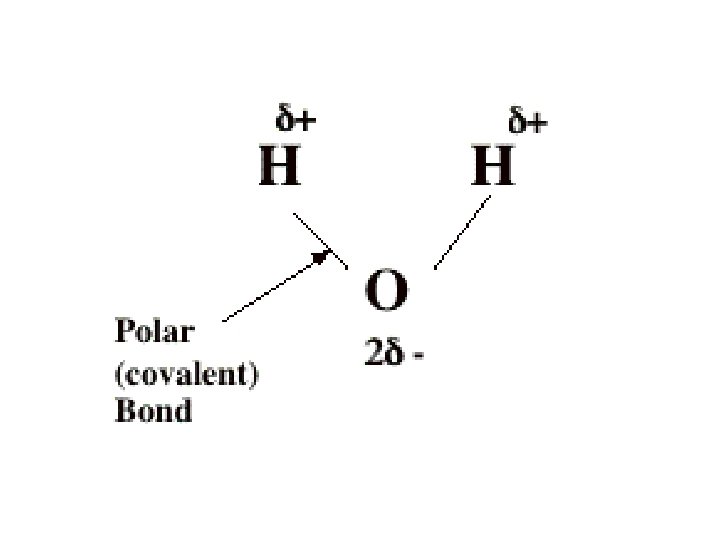

Hydrogen bonding l Occurs due to polar-covalent bonding in a molecule l The slightly + hydrogen atom of one molecule is attracted to the slightly – oxygen atom in another molecule these bonds are weak on their own, but them together are strong think velcro! lots of
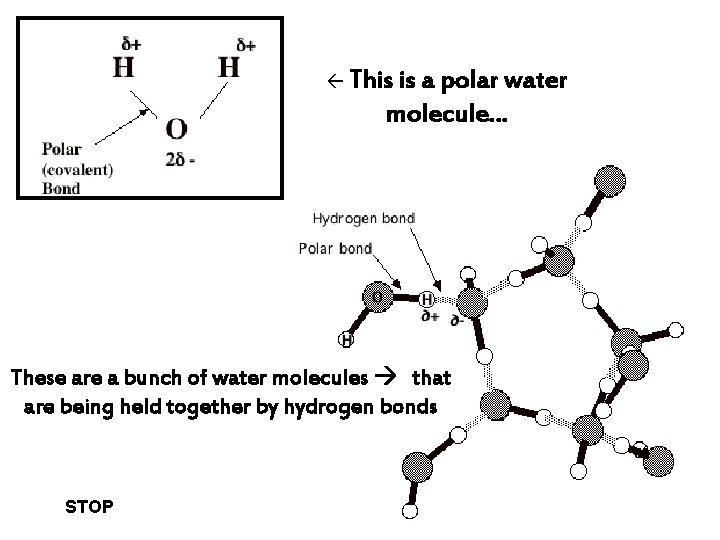
This is a polar water molecule… These are a bunch of water molecules that are being held together by hydrogen bonds STOP
2. 3 Chemistry of Water PROPERTIES: l l l High heat capacity – many hydrogen bonds help to absorb heat High heat of vaporization Water is a solvent – other polar molecules will dissolve easily in it Water molecules are cohesive and adhesive – it flows easily and sticks to other polar substances Water has a high surface tension Ice is less dense than water – the spaces between the molecules in solid water are farther apart than in their liquid state!
Acids and bases l Acids release H+ o They increase the hydrogen ion concentration and decrease p. H l Bases release OH- or accept H+ o They decrease the hydrogen ion concentration and increase p. H l p. H scale ranges from 0 -14 l Buffer = a chemical that keeps p. H levels stable
Organic molecules Always contain Carbon and Hydrogen! ps: carbon can only bond to 4 things at once l Carbohydrates, lipids, proteins and nucleic acids are organic molecules with specific functions in cells l Carbohydrates, proteins and nucleic acids are polymers (long chains of monomers linked together by chemical bonds) l FUNCTIONAL GROUP = a group of atoms that always behaves the same way l
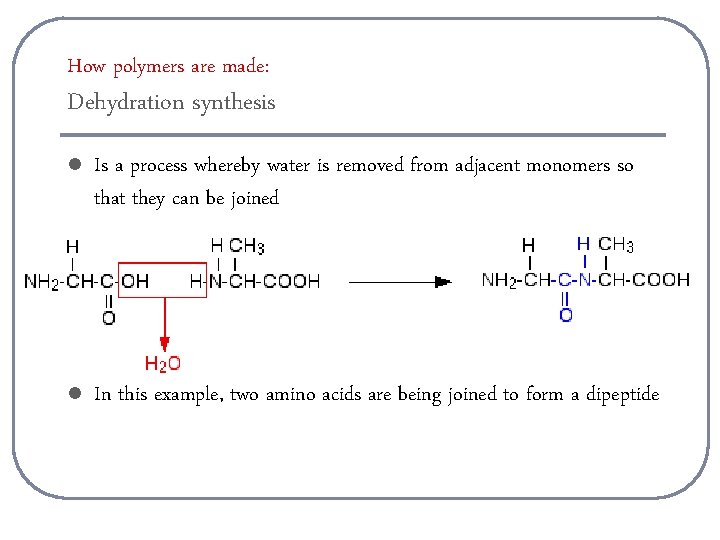
How polymers are made: Dehydration synthesis l Is a process whereby water is removed from adjacent monomers so that they can be joined l In this example, two amino acids are being joined to form a dipeptide
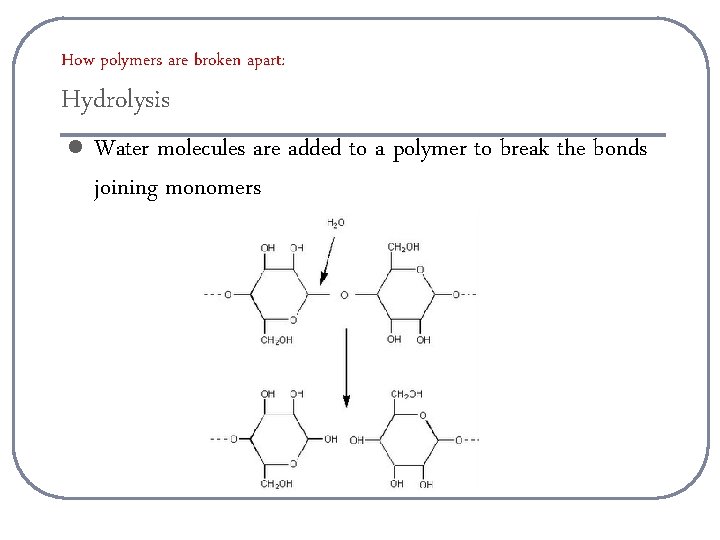
How polymers are broken apart: Hydrolysis l Water molecules are added to a polymer to break the bonds joining monomers
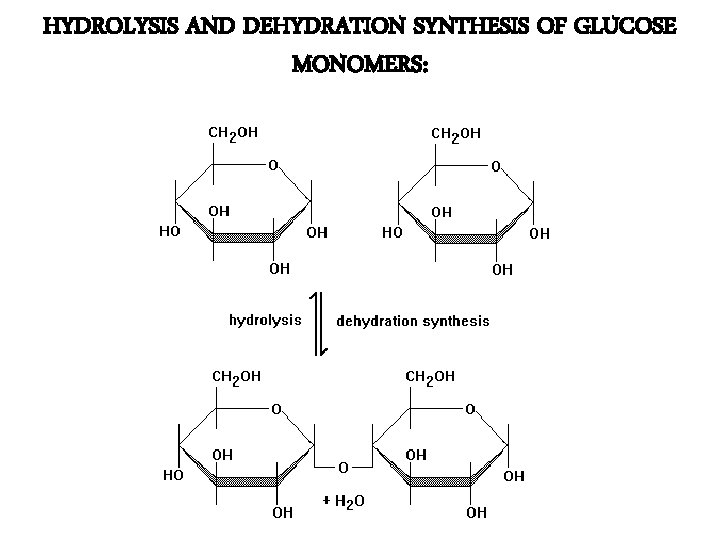
HYDROLYSIS AND DEHYDRATION SYNTHESIS OF GLUCOSE MONOMERS:
2. 5 Carbohydrates l Function for quick fuel and short-term energy storage in all organisms l Characterized by the presence of the atomic grouping H-C-OH Note the ratio of C to H to O is approximately 1: 2: 1 Cn. H 2 n. On
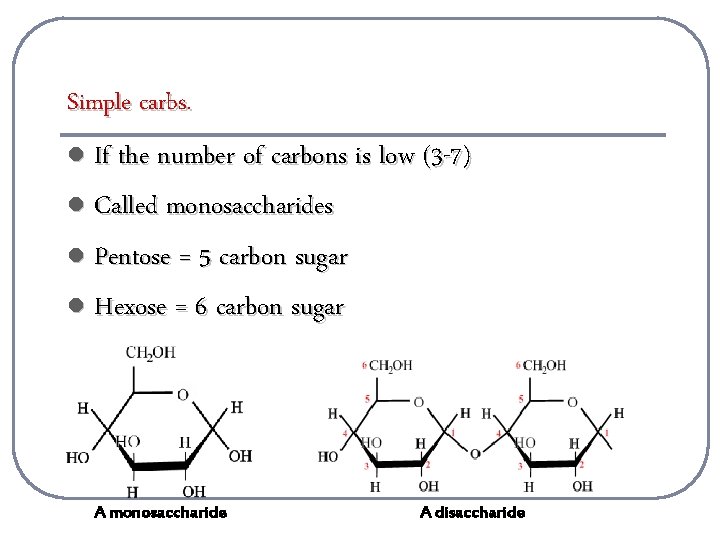
Simple carbs. l If the number of carbons is low (3 -7) l Called monosaccharides l Pentose = 5 carbon sugar l Hexose = 6 carbon sugar A monosaccharide A disaccharide
The 3 hexoses… l Glucose, fructose and galactose l All three hexoses occur as ring structures with the molecular formula: C 6 H 12 O 6 l The exact shape of the ring, and the placement of hydrogen (H) and hydroxyl (-OH) groups are the only differences!
Glucose… l l Is a hexose AKA: blood sugar or dextrose It is important that you can draw the structure of alpha glucose and number the C atoms in the ring correctly! The numbers on the C atoms are important when molecules of glucose are linked together via a chemical bond
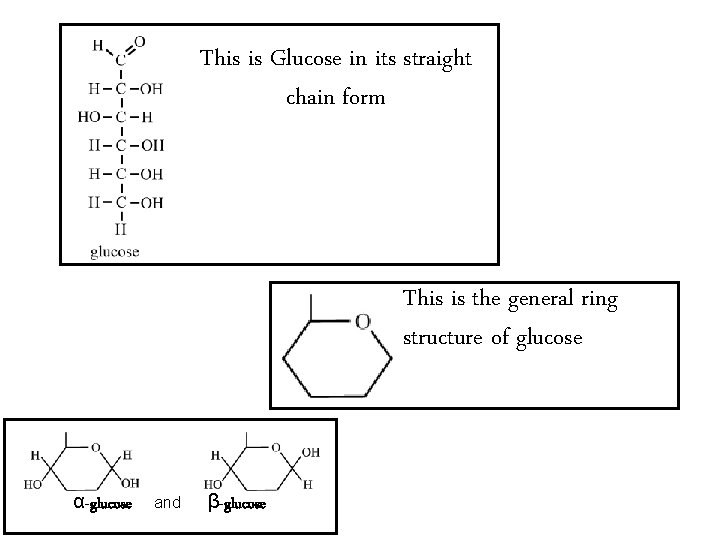
This is Glucose in its straight chain form This is the general ring structure of glucose α-glucose and β-glucose
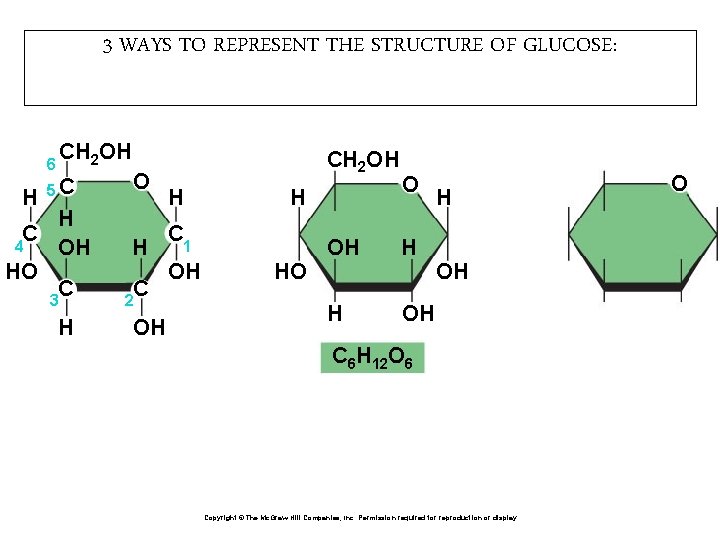
3 WAYS TO REPRESENT THE STRUCTURE OF GLUCOSE: 6 H CH 2 OH 5 C H C 4 OH HO C 3 H O H C 2 OH CH 2 OH H H C 1 OH HO O OH H H OH C 6 H 12 O 6 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. O
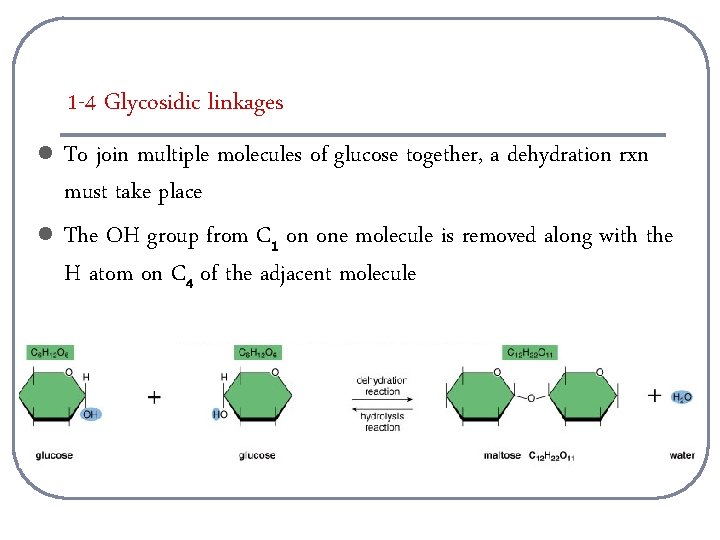
1 -4 Glycosidic linkages l l To join multiple molecules of glucose together, a dehydration rxn must take place The OH group from C 1 on one molecule is removed along with the H atom on C 4 of the adjacent molecule
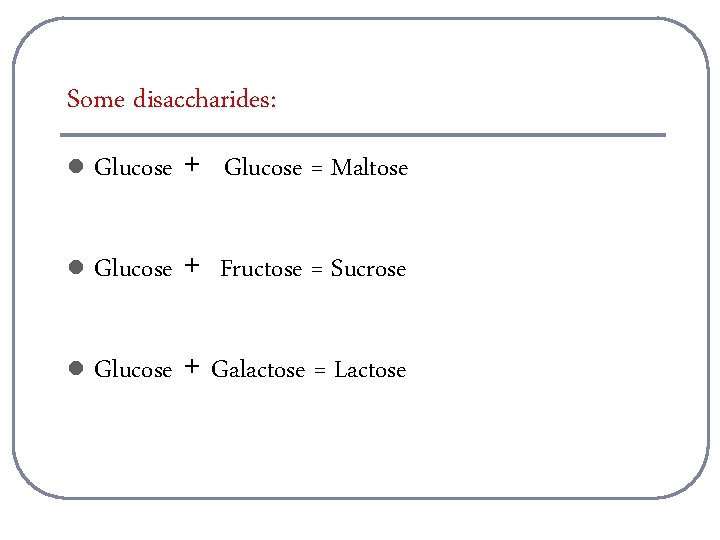
Some disaccharides: l Glucose + Glucose = Maltose l Glucose + Fructose = Sucrose l Glucose + Galactose = Lactose
Polysaccharides AKA: Complex carbohydrates l Long polymers that contain many glucose subunits l Starch, glycogen, and cellulose are examples l Starch and glycogen are ready storage forms of glucose in plants and animals, respectively l Cellulose is found in plant cell walls l
Starch l l Some polymers of starch are long chains of up to 4000 glucose units Starch has fewer side branches off the main chain than glycogen Once we eat starchy foods, our bodies break it down into glucose units The glucose enters our bloodstream, and then the liver stores it as glycogen
Glycogen l Storage form of glucose in animals l Recognizable highly-branched structure l This polysaccharide accumulates in the liver and small amounts in muscle tissue l The liver releases glycogen so that glucose concentration is always about 0. 1%
Cellulose A structural polysaccharide l Found in the plant cell walls l l o This accounts for the strong nature of these walls In cellulose, the glucose units are joined by a different type of linkage o The position of the oxygen atoms alternates! This is significant, because some animals cannot digest foods that contain this linkage l. : it passes through our system fiber/roughage l
2. 6 Lipids Fats and oils function as energy storage molecules in organisms l Phospholipids are the basis of cell membranes l Steroids are a large class of lipids that includes the sex hormones. . . l All types of lipids do not dissolve in water! l
Fats and oils l Fats are solids at room temperature usually saturated and of animal origin long term energy; insulates against heat loss; forms protective cushion around organs Oils are liquids at room temperature usually unsaturated and of plant origin
Formation… l Fats and oils form when one glycerol molecule reacts with 3 fatty acid molecules l Fats are sometimes called triglycerides l The term NEUTRAL FAT is sometimes used because the molecule is non-polar
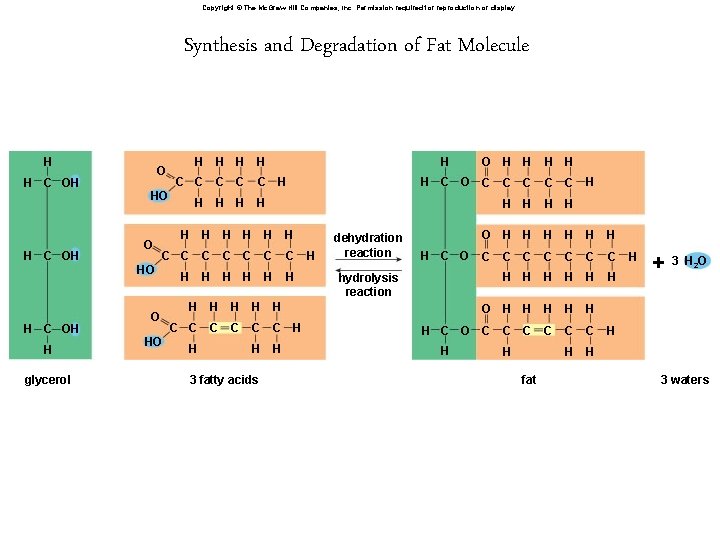
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Synthesis and Degradation of Fat Molecule H H C OH O O H glycerol H H C C C C HO H H H H H C C C C H H H O HO H H H C C C H H H O H H H C O C H HO H C OH H 3 fatty acids H H dehydration reaction H C O C C H H H O H H H C C C H H H hydrolysis reaction H C O C H H H C C H fat H +3 HO 2 3 waters

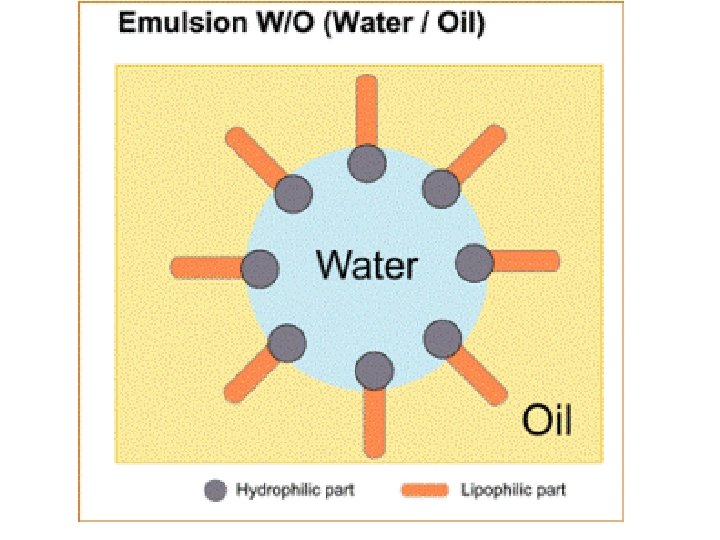
Emulsification Emulsifiers cause fats to mix with water l They contain molecules with both polar and non-polar ends l The molecules surround droplets of fat so that their nonpolar end project inward, and their polar end outward l This way, the droplet can disperse in the water dish soap bile (in your digestive tract) l
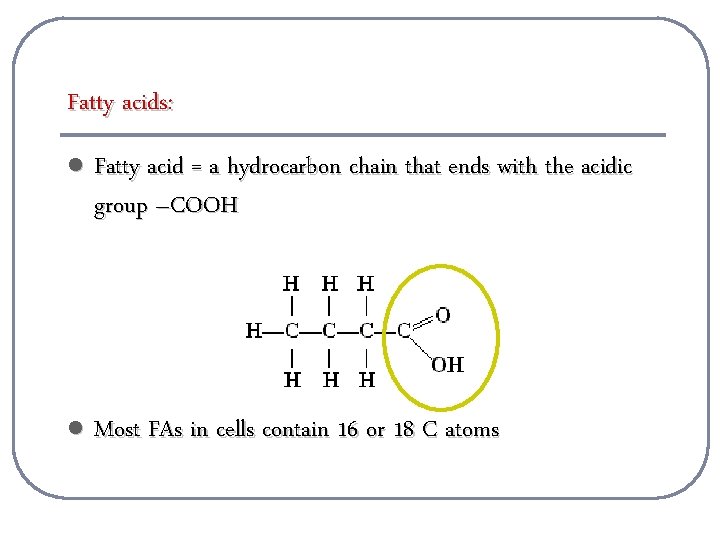
Fatty acids: l Fatty acid = a hydrocarbon chain that ends with the acidic group –COOH l Most FAs in cells contain 16 or 18 C atoms
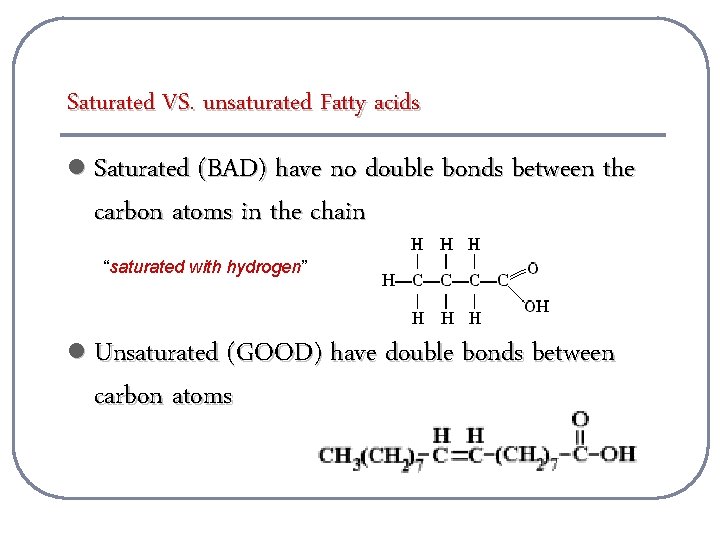
Saturated VS. unsaturated Fatty acids l Saturated (BAD) have no double bonds between the carbon atoms in the chain “saturated with hydrogen” l Unsaturated (GOOD) have double bonds between carbon atoms
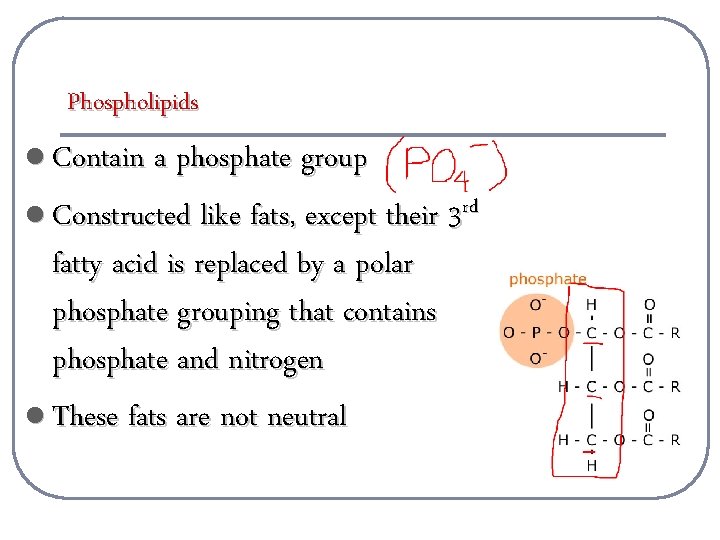
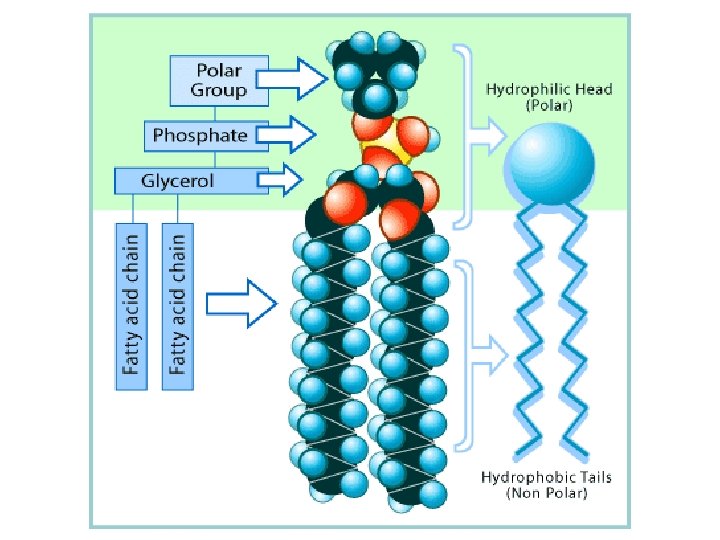
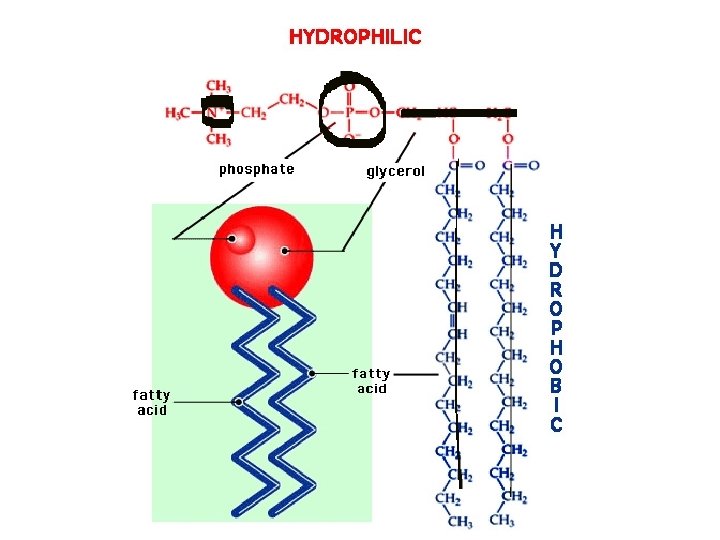
Phospholipids l Contain a phosphate group l Constructed like fats, except their 3 rd fatty acid is replaced by a polar phosphate grouping that contains phosphate and nitrogen l These fats are not neutral
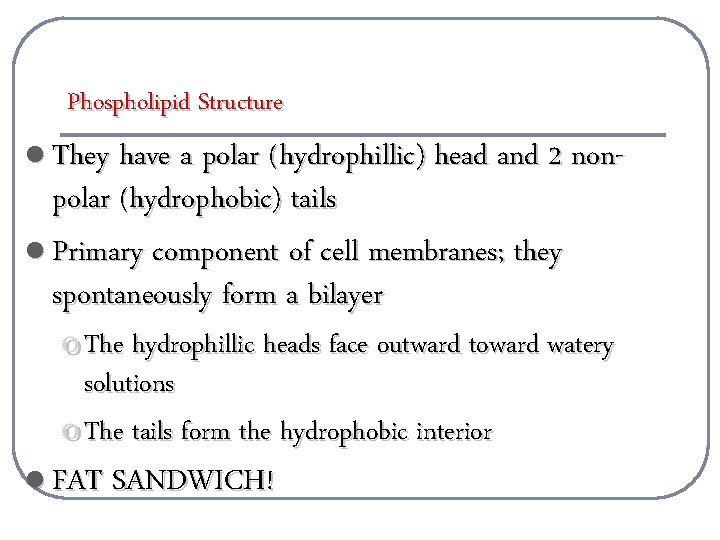
Phospholipid Structure l They have a polar (hydrophillic) head and 2 non- polar (hydrophobic) tails l Primary component of cell membranes; they spontaneously form a bilayer o The hydrophillic heads face outward toward watery solutions o The tails form the hydrophobic interior l FAT SANDWICH!
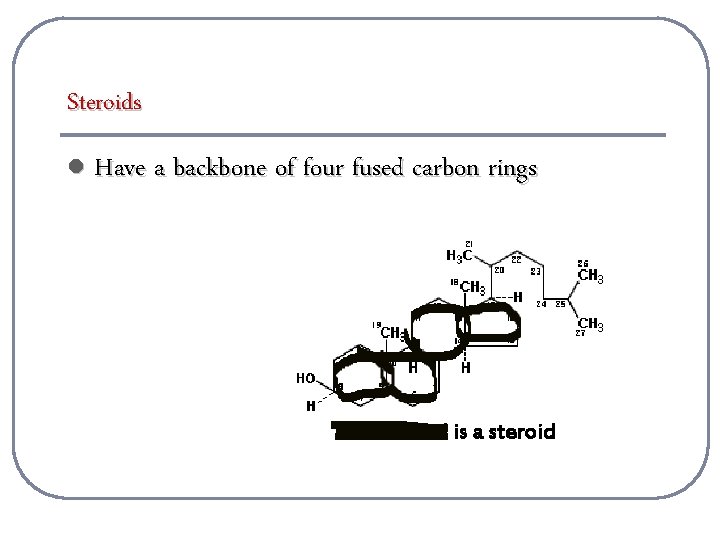
Steroids l Have a backbone of four fused carbon rings Cholesterol is a steroid
Cholesterol l Steroid formed by the body and also part of our diet Has several important functions o Component of animal cell’s membrane o Precursor to other steroids, including bile salts and testosterone and estrogen Diets high in saturated fats, trans fats and cholesterol can lead to the accumulation of fatty material inside blood vessels. STOP
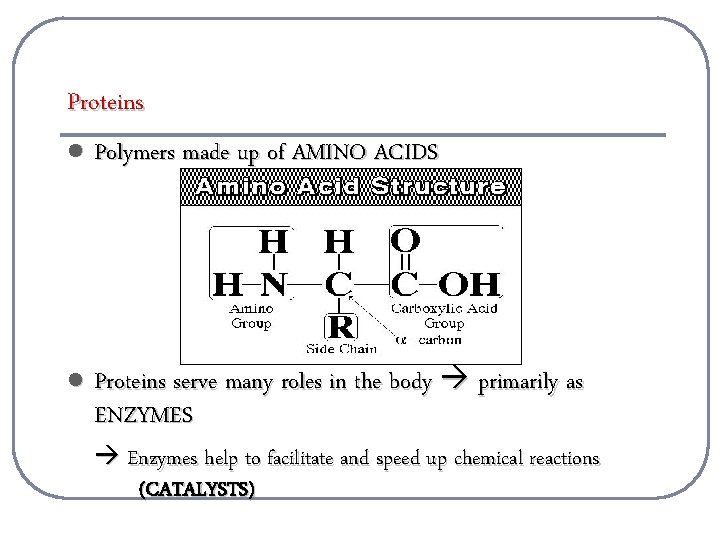
Proteins l Polymers made up of AMINO ACIDS l Proteins serve many roles in the body primarily as ENZYMES Enzymes help to facilitate and speed up chemical reactions (CATALYSTS)
Other functions: l Collagen and keratin (hair and nails) l Some hormones are protein-based l Actin and myosin are proteins involved in cell movement and muscle contraction l Hemoglobin is a protein in our blood that transports oxygen l Antibodies within our immune system l Proteins within cell membranes help to move materials back and forth
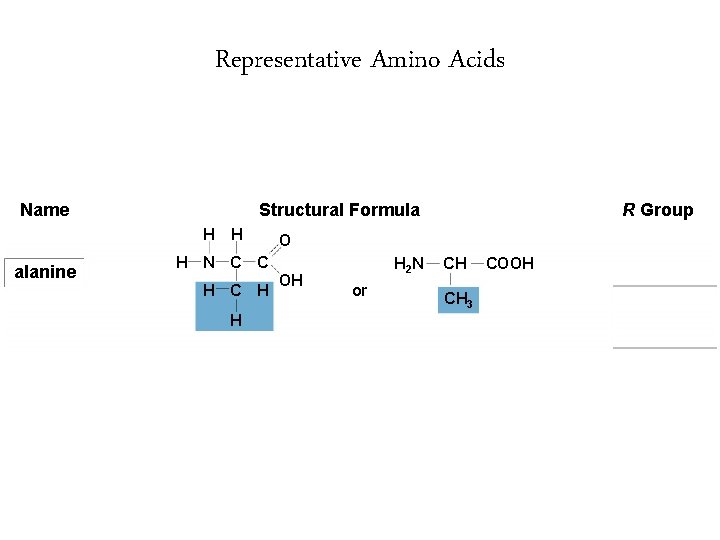
Representative Amino Acids Name Structural Formula H alanine H O H N C C H H R Group OH H 2 N or CH CH 3 COOH
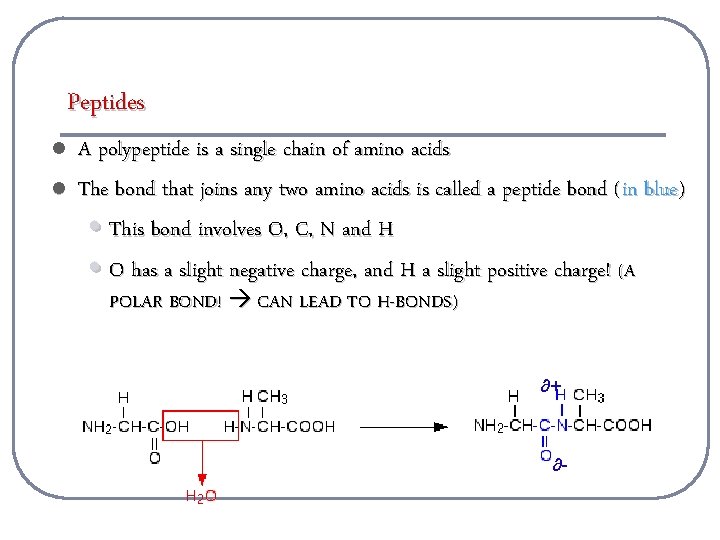
Peptides l l A polypeptide is a single chain of amino acids The bond that joins any two amino acids is called a peptide bond ( in blue) • This bond involves O, C, N and H • O has a slight negative charge, and H a slight positive charge! (A POLAR BOND! CAN LEAD TO H-BONDS) ∂+ ∂-
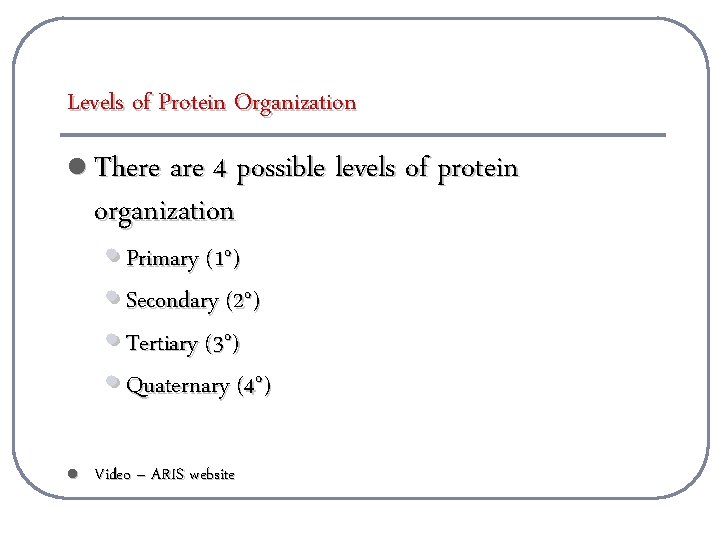
Levels of Protein Organization l There are 4 possible levels of protein organization • Primary (1 o) • Secondary (2 o) • Tertiary (3 o) • Quaternary (4 o) l Video – ARIS website
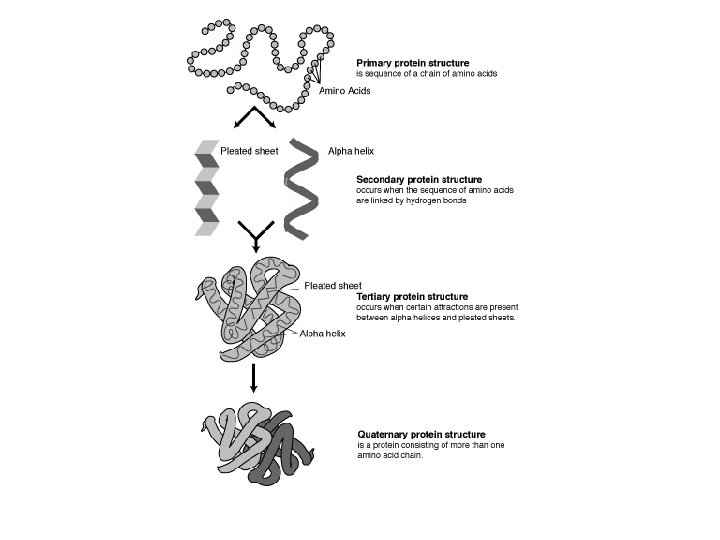
Primary Structure l A linear sequence of amino acids l Like beads on a string l This level of structure is determined by the sequence of a. a. ’s that join to form the polypeptide. l The sequence in this form will also determine whether the protein will fold/coil up further to 2 o or 3 o degrees. . . l All proteins have a 1 o structure!
Secondary Structure l Hydrogen bonding occurs between adjacent amino acids in the polypeptide l This can result in 2 spatial orientations: • Alpha-helices (right-handed spirals) or coils • Beta-pleated sheets (folding)
Tertiary structure l l l When further bonding takes place between amino acids in the chain a more complex 3 D shape can take place In addition to H-bonds, ionic and covalent bonds (between adjacent Rgroups) can cause the polypeptide to fold up on itself even more • Bonds such as disulphide linkage (between two S atoms) This is a protein’s final 3 D shape Often, hydrophobic regions of the chain will fold inwards, and hydrophillic regions outwards Globular proteins all have a 3 o structure
Quaternary Structure l When 2 or more polypeptide chains combine to form a single protein • Hemoglobin and most enzymes have a 4 o structure! These different levels of organization is what makes each protein chemically and structurally unique! l The final shape of any protein is crucial when it comes to its function l When proteins are exposed to extremes in heat and p. H, they undergo a irreversible change called DENATURATION l
2. 8 Nucleic Acids TWO TYPES: l Deoxyribonucleic acid (DNA) l Ribonucleic acid (RNA) l Both are polymers (long chains) of nucleotides
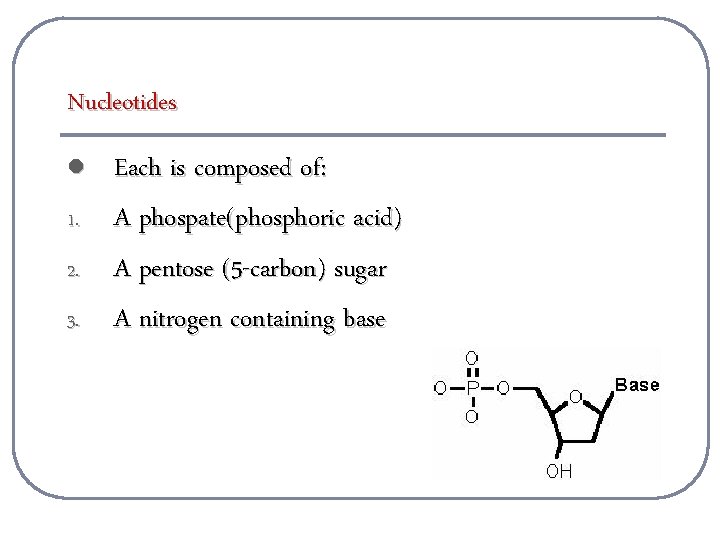
Nucleotides l 1. 2. 3. Each is composed of: A phospate(phosphoric acid) A pentose (5 -carbon) sugar A nitrogen containing base
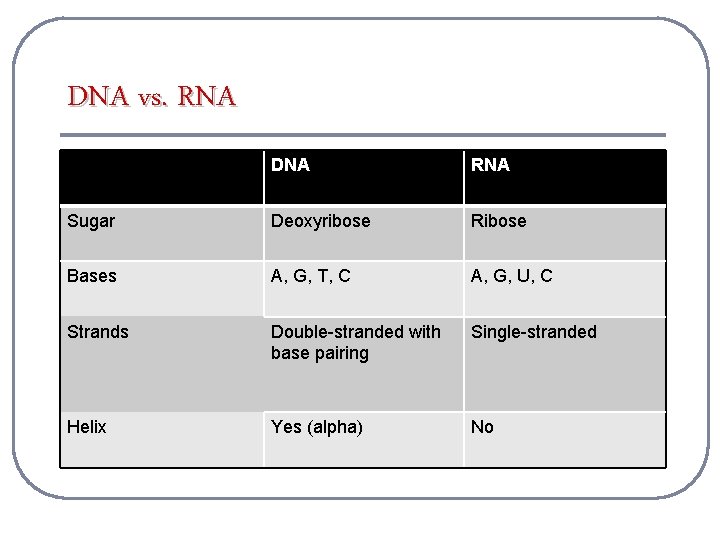
DNA vs. RNA DNA RNA Sugar Deoxyribose Ribose Bases A, G, T, C A, G, U, C Strands Double-stranded with base pairing Single-stranded Helix Yes (alpha) No
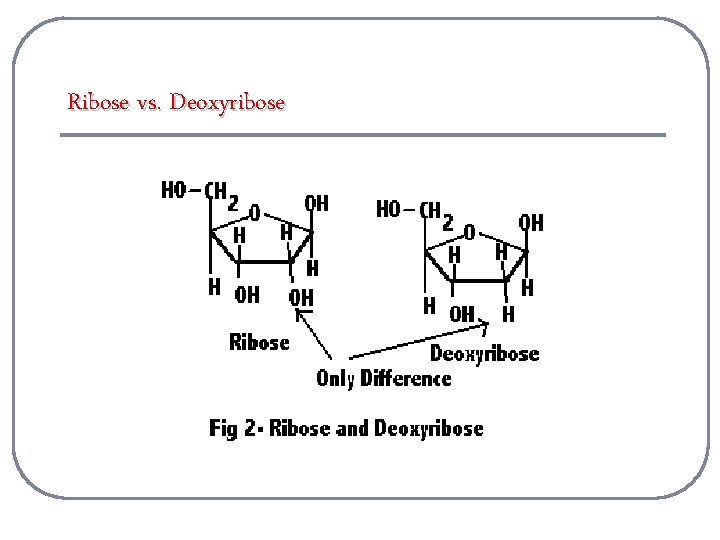
Ribose vs. Deoxyribose
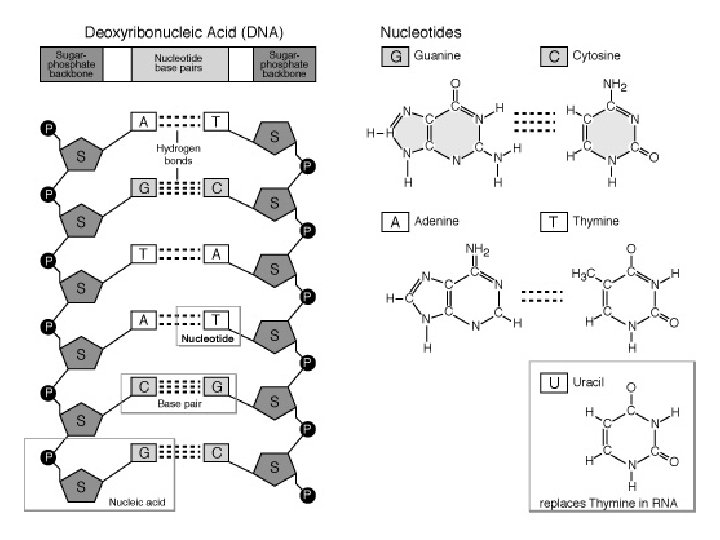
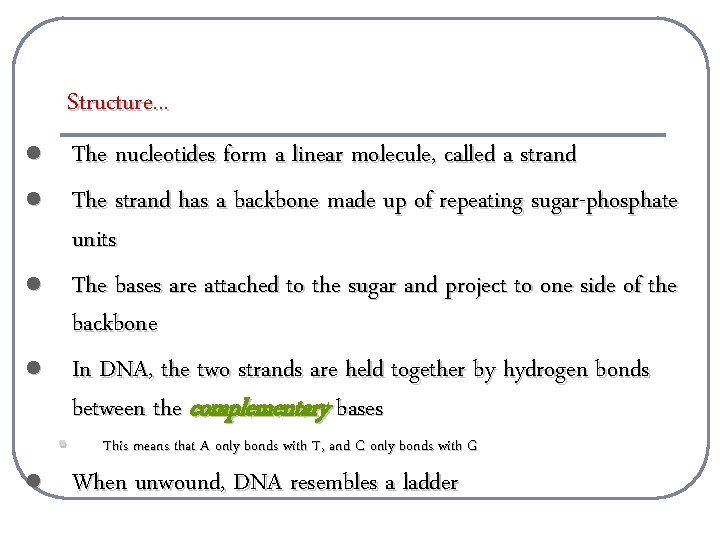
Structure. . . l l l • The nucleotides form a linear molecule, called a strand The strand has a backbone made up of repeating sugar-phosphate units The bases are attached to the sugar and project to one side of the backbone In DNA, the two strands are held together by hydrogen bonds between the complementary bases This means that A only bonds with T, and C only bonds with G When unwound, DNA resembles a ladder
Structure. . . l l Complementary base pairing ensures that DNA can be “unzipped” and each strand can be used as a template to copy itself exactly DNA’s sequence contains a code that specifies the sequence of amino acids in the proteins of the cell! RNA l l RNA is formed by complementary base pairing with one DNA strand m. RNA carries the information from the DNA strand to a ribosome, where the code is translated into the amino acid sequence!
ATP: Adenosine Triphosphate l l l When adenosine (adenine + ribose) is modified by the addition of 3 phosphate groups –instead of one – it becomes ATP This is the energy molecule of the cell! ATP is a high-energy molecule because the last 2 phosphate bonds are unstable and easily broken When the terminal phosphate bond is hydrolyzed it releases a lot of energy: ATP ADP + phosphate + energy This is the energy used throughout the cell for pretty much everything. . .
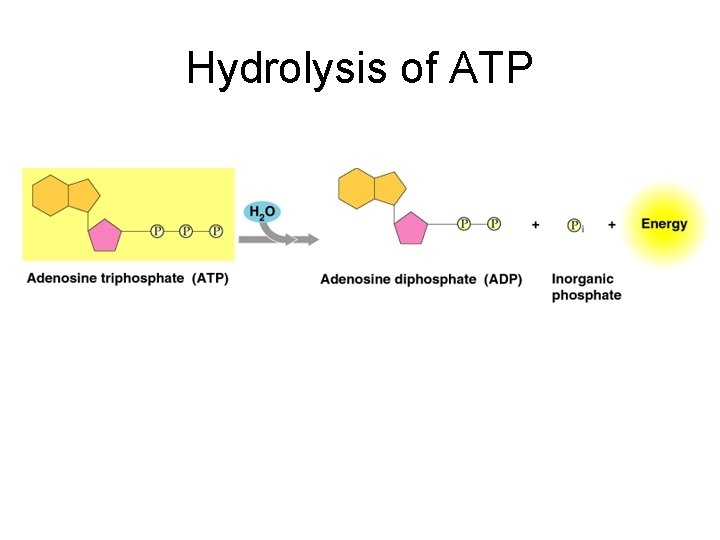
Hydrolysis of ATP

Your assignment: Finish your “Molecules of the cell” package l Make cue cards for the remainder of the B 4 PLOs l Do the online quiz on the ARIS website! l Study for your first Chapter Test on Monday: l • Reread your notes • USE your CUE CARDS!!! • Read the textbook – do the chapter assessment • Look at the PLOs and your vocab
- Slides: 70