The Mole Unit 5 Formula Mass Formula mass


- Slides: 16

The Mole Unit 5

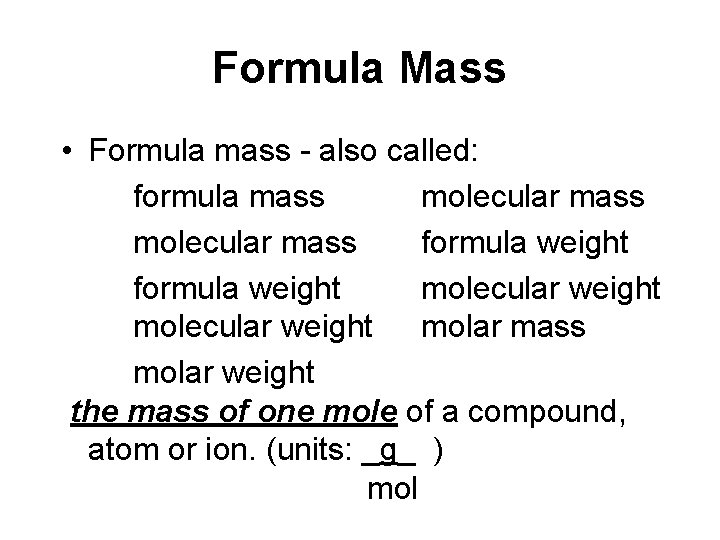
Formula Mass • Formula mass - also called: formula mass molecular mass formula weight molecular weight molar mass molar weight the mass of one mole of a compound, atom or ion. (units: _g_ ) mol

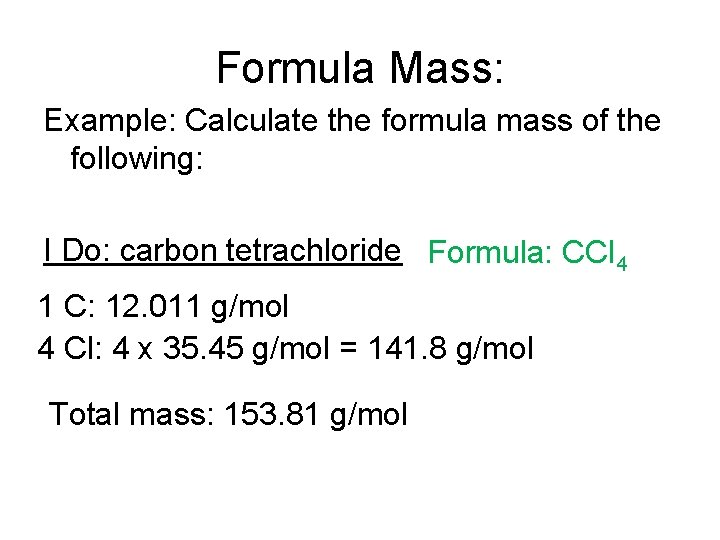
Formula Mass: Example: Calculate the formula mass of the following: I Do: carbon tetrachloride Formula: CCl 4 1 C: 12. 011 g/mol 4 Cl: 4 x 35. 45 g/mol = 141. 8 g/mol Total mass: 153. 81 g/mol

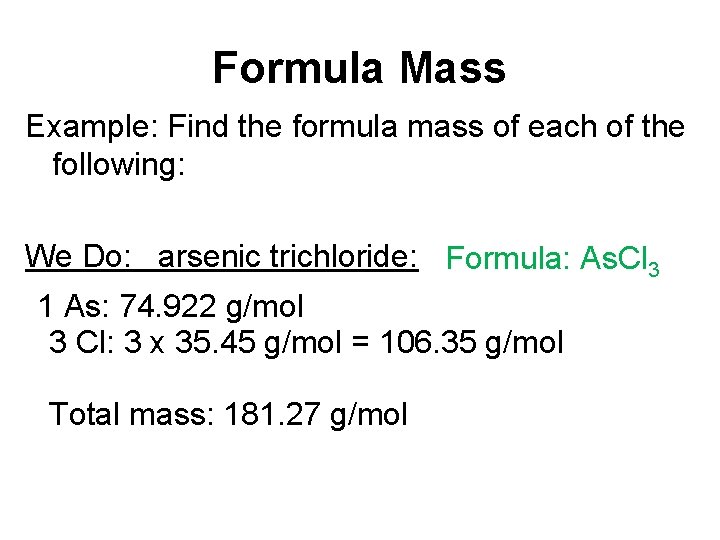
Formula Mass Example: Find the formula mass of each of the following: We Do: arsenic trichloride: Formula: As. Cl 3 1 As: 74. 922 g/mol 3 Cl: 3 x 35. 45 g/mol = 106. 35 g/mol Total mass: 181. 27 g/mol

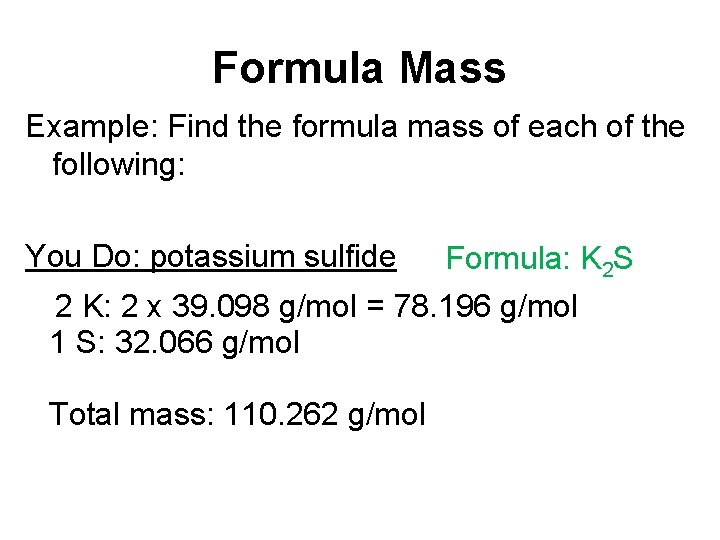
Formula Mass Example: Find the formula mass of each of the following: You Do: potassium sulfide Formula: K 2 S 2 K: 2 x 39. 098 g/mol = 78. 196 g/mol 1 S: 32. 066 g/mol Total mass: 110. 262 g/mol

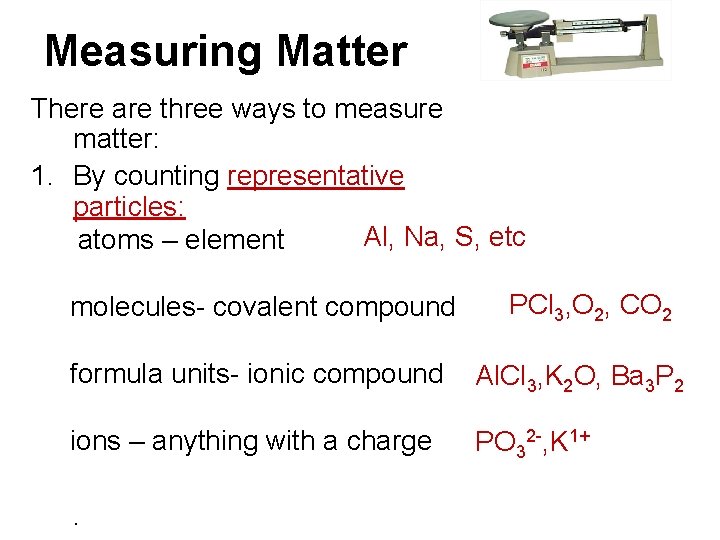
Measuring Matter There are three ways to measure matter: 1. By counting representative particles: Al, Na, S, etc atoms – element molecules- covalent compound PCl 3, O 2, CO 2 formula units- ionic compound Al. Cl 3, K 2 O, Ba 3 P 2 ions – anything with a charge PO 32 -, K 1+ .

2. By mass - in grams 3. By volume - in liters for gases (at STP)

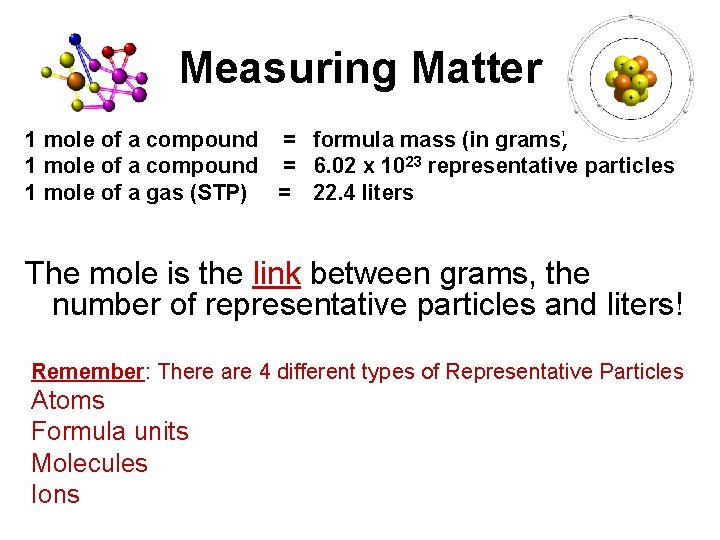
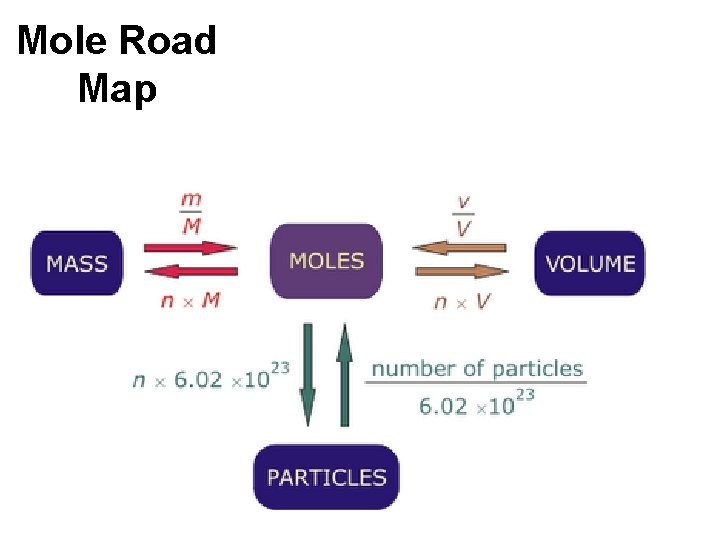
Measuring Matter 1 mole of a compound = formula mass (in grams) 1 mole of a compound = 6. 02 x 1023 representative particles 1 mole of a gas (STP) = 22. 4 liters The mole is the link between grams, the number of representative particles and liters! Remember: There are 4 different types of Representative Particles Atoms Formula units Molecules Ions

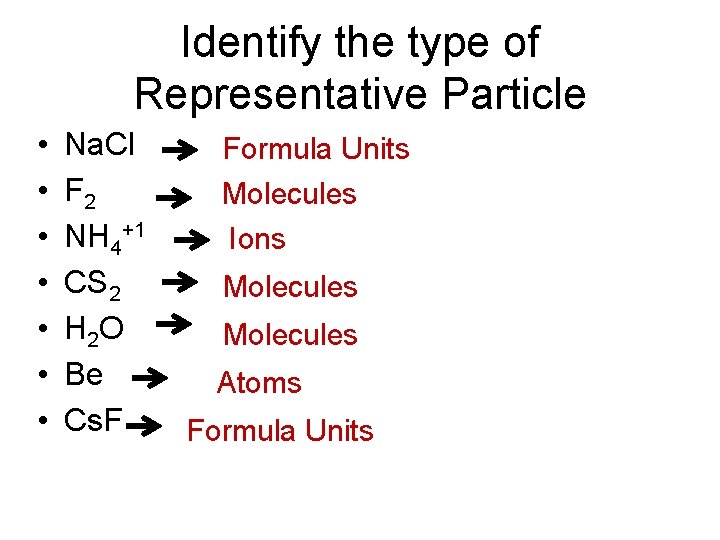
Identify the type of Representative Particle • • Na. Cl F 2 NH 4+1 CS 2 H 2 O Be Cs. F Formula Units Molecules Ions Molecules Atoms Formula Units

Mole Road Map

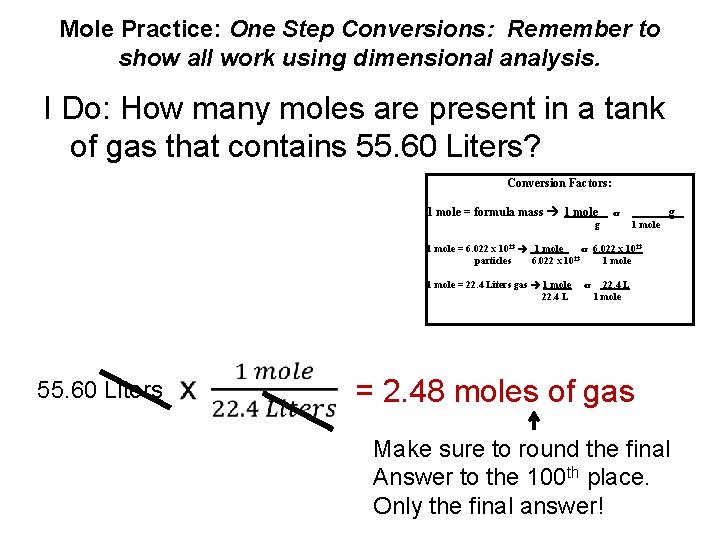
Mole Practice: One Step Conversions: Remember to show all work using dimensional analysis. I Do: How many moles are present in a tank of gas that contains 55. 60 Liters? Conversion Factors: 1 mole = formula mass 1 mole g or g 1 mole = 6. 022 x 1023 1 mole or 6. 022 x 1023 23 particles 6. 022 x 10 1 mole = 22. 4 Liters gas 1 mole 22. 4 L 55. 60 Liters or 22. 4 L 1 mole = 2. 48 moles of gas Make sure to round the final Answer to the 100 th place. Only the final answer!

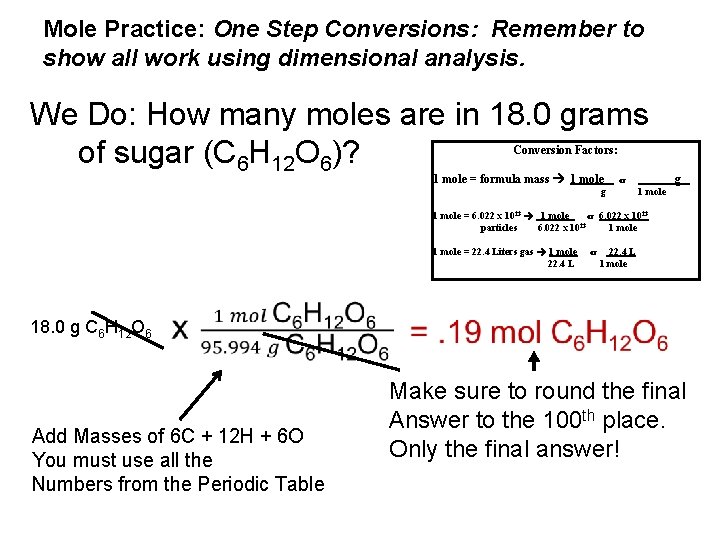
Mole Practice: One Step Conversions: Remember to show all work using dimensional analysis. We Do: How many moles are in 18. 0 grams of sugar (C 6 H 12 O 6)? Conversion Factors: 1 mole = formula mass 1 mole or g g 1 mole = 6. 022 x 1023 1 mole or 6. 022 x 1023 particles 6. 022 x 1023 1 mole = 22. 4 Liters gas 1 mole 22. 4 L 18. 0 g C 6 H 12 O 6 Add Masses of 6 C + 12 H + 6 O You must use all the Numbers from the Periodic Table or 22. 4 L 1 mole Make sure to round the final Answer to the 100 th place. Only the final answer!

Mole Practice: One Step Conversions: Remember to show all work using dimensional analysis. You Do: What is the mass in grams of 4. 50 moles of barium sulfide? Conversion Factors: 1 mole = formula mass 1 mole Add Masses of 1 Ba + 1 S You must use all the Numbers from the Periodic Table 4. 50 mol Ba. S g or g 1 mole = 6. 022 x 1023 1 mole or 6. 022 x 1023 23 particles 6. 022 x 10 1 mole = 22. 4 Liters gas 1 mole 22. 4 L or 22. 4 L 1 mole Make sure to round the final Answer to the 100 th place. Only the final answer!

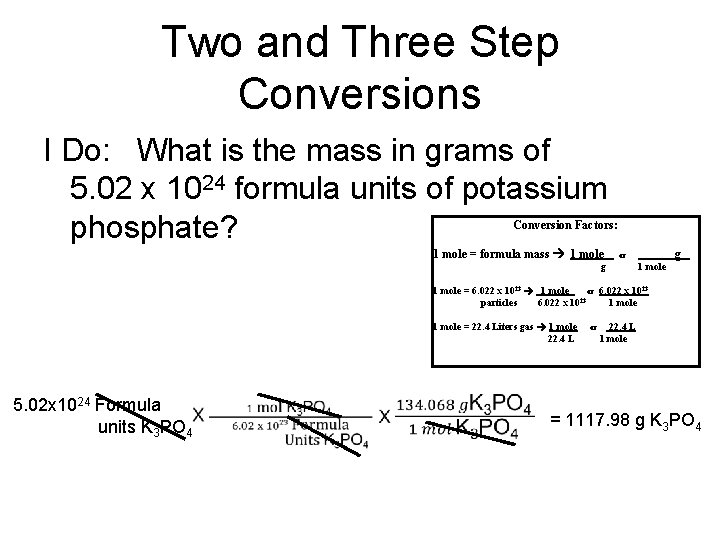
Two and Three Step Conversions I Do: What is the mass in grams of 5. 02 x 1024 formula units of potassium phosphate? Conversion Factors: 1 mole = formula mass 1 mole g or g 1 mole = 6. 022 x 1023 1 mole or 6. 022 x 1023 23 particles 6. 022 x 10 1 mole = 22. 4 Liters gas 1 mole 22. 4 L 5. 02 x 1024 Formula units K 3 PO 4 or 22. 4 L 1 mole = 1117. 98 g K 3 PO 4

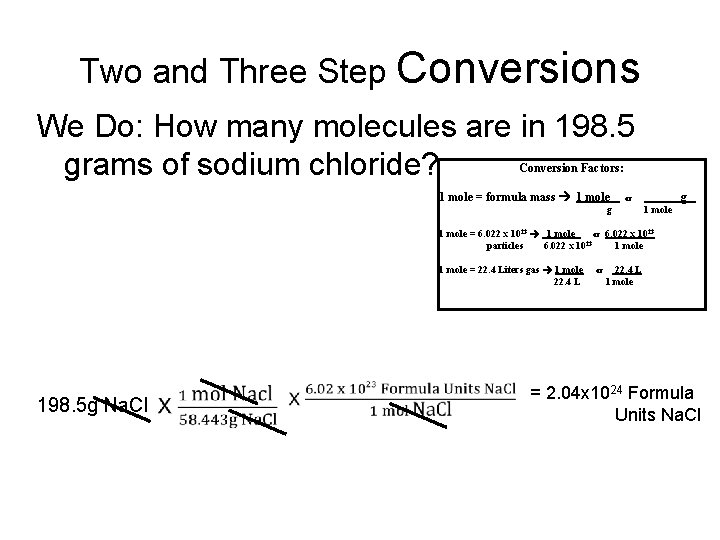
Two and Three Step Conversions We Do: How many molecules are in 198. 5 grams of sodium chloride? Conversion Factors: 1 mole = formula mass 1 mole g or g 1 mole = 6. 022 x 1023 1 mole or 6. 022 x 1023 23 particles 6. 022 x 10 1 mole = 22. 4 Liters gas 1 mole 22. 4 L 198. 5 g Na. Cl or 22. 4 L 1 mole = 2. 04 x 1024 Formula Units Na. Cl

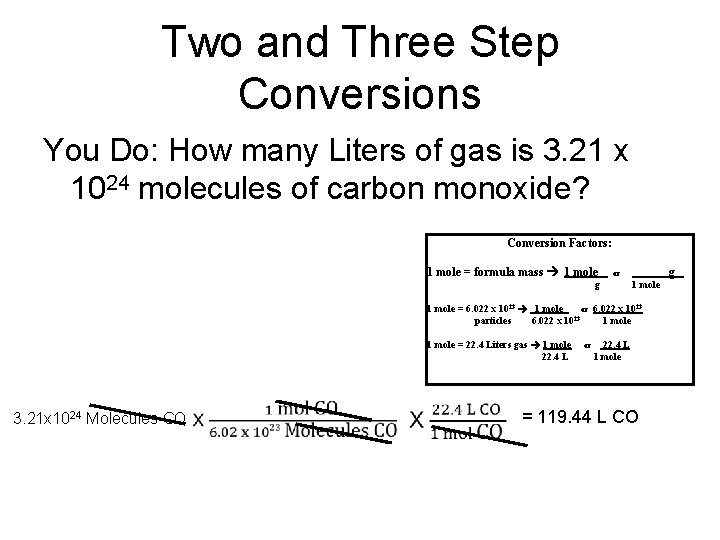
Two and Three Step Conversions You Do: How many Liters of gas is 3. 21 x 1024 molecules of carbon monoxide? Conversion Factors: 1 mole = formula mass 1 mole g or g 1 mole = 6. 022 x 1023 1 mole or 6. 022 x 1023 23 particles 6. 022 x 10 1 mole = 22. 4 Liters gas 1 mole 22. 4 L 3. 21 x 1024 Molecules CO or 22. 4 L 1 mole = 119. 44 L CO