The Mole The mole is a counting unit


- Slides: 16


The Mole • The mole is a counting unit in chemistry. • Abbreviated mol. • Defined as the number equal to the number of carbon atoms in exactly 12 grams of pure 12 C. • Has the value 6. 022 x 1023. • Called Avogradro’s number to honor his contributions to chemistry. • One mole of anything consists of 6. 022 x 1023 units of that substance. • Just like a dozen eggs is 12 eggs; a mole of eggs is 6. 022 x 1023 eggs.

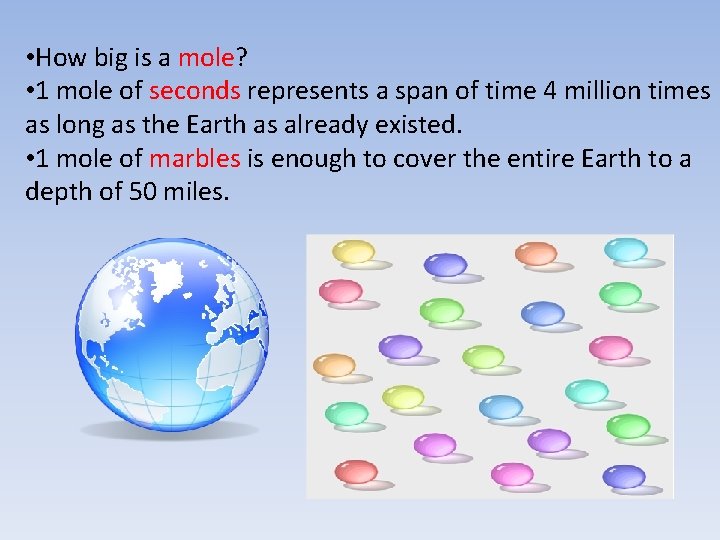
• How big is a mole? • 1 mole of seconds represents a span of time 4 million times as long as the Earth as already existed. • 1 mole of marbles is enough to cover the entire Earth to a depth of 50 miles.

• Remember that the mole is the number of atoms in exactly 12 grams of 12 C therefore 12 grams of 12 C contains exactly 6. 022 x 1023 atoms. • Plus it tells us that a 12. 01 gram sample of natural carbon contains 6. 022 x 1023 atoms (natural carbon is a mixture of the isotopes of carbon). • Notice that the value 12. 01 grams of natural carbon is the same as the atomic mass value (12. 01 amu). • It also tells us that 26. 98 grams of aluminum contains exactly 6. 022 x 1023 atoms of aluminum.

• If a mole of natural carbon and aluminum have the same number of atoms why do they have different masses? • Consider a dozen doughnuts and a dozen tires. • Would they have the same mass? • We can also define the mole such that a sample of a natural element with a mass equal to the element’s atomic mass expressed in grams contains 1 mole of atoms.


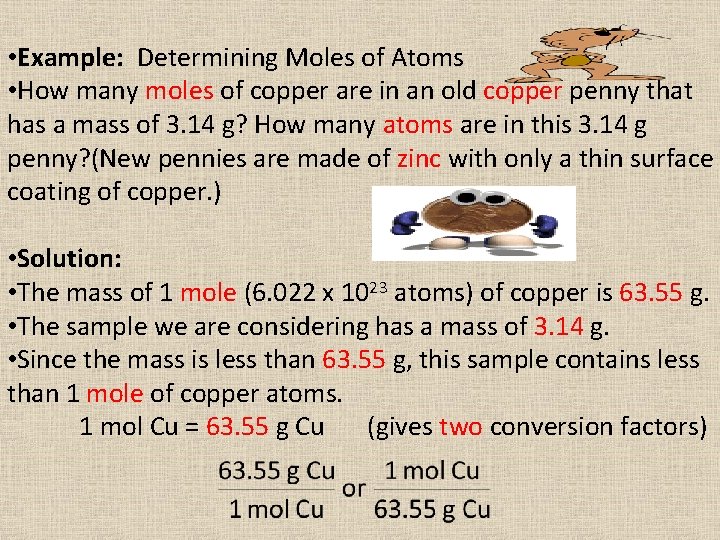
• Example: Determining Moles of Atoms • How many moles of copper are in an old copper penny that has a mass of 3. 14 g? How many atoms are in this 3. 14 g penny? (New pennies are made of zinc with only a thin surface coating of copper. ) • Solution: • The mass of 1 mole (6. 022 x 1023 atoms) of copper is 63. 55 g. • The sample we are considering has a mass of 3. 14 g. • Since the mass is less than 63. 55 g, this sample contains less than 1 mole of copper atoms. 1 mol Cu = 63. 55 g Cu (gives two conversion factors)

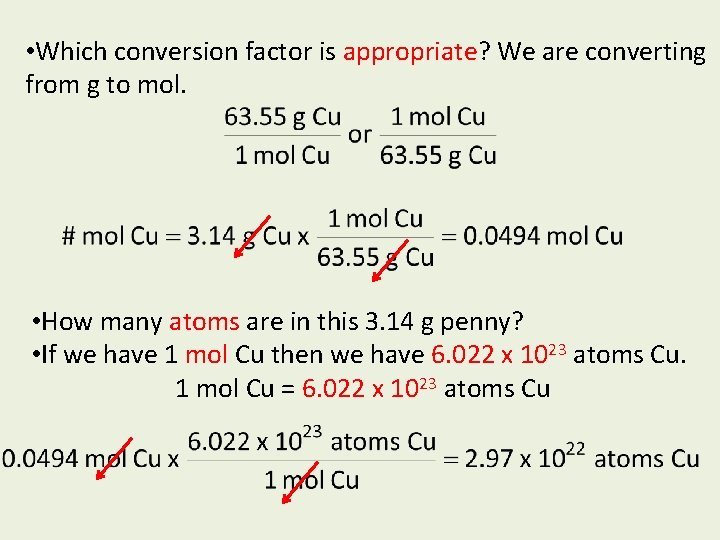
• Which conversion factor is appropriate? We are converting from g to mol. • How many atoms are in this 3. 14 g penny? • If we have 1 mol Cu then we have 6. 022 x 1023 atoms Cu. 1 mol Cu = 6. 022 x 1023 atoms Cu

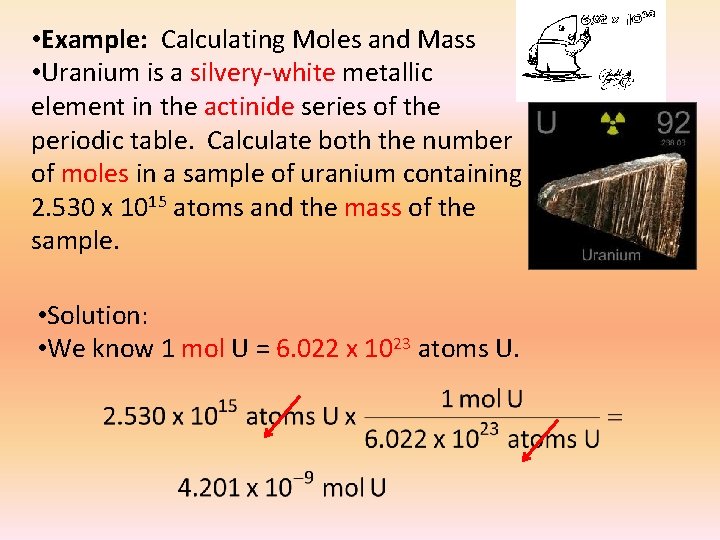
• Example: Calculating Moles and Mass • Uranium is a silvery-white metallic element in the actinide series of the periodic table. Calculate both the number of moles in a sample of uranium containing 2. 530 x 1015 atoms and the mass of the sample. • Solution: • We know 1 mol U = 6. 022 x 1023 atoms U.

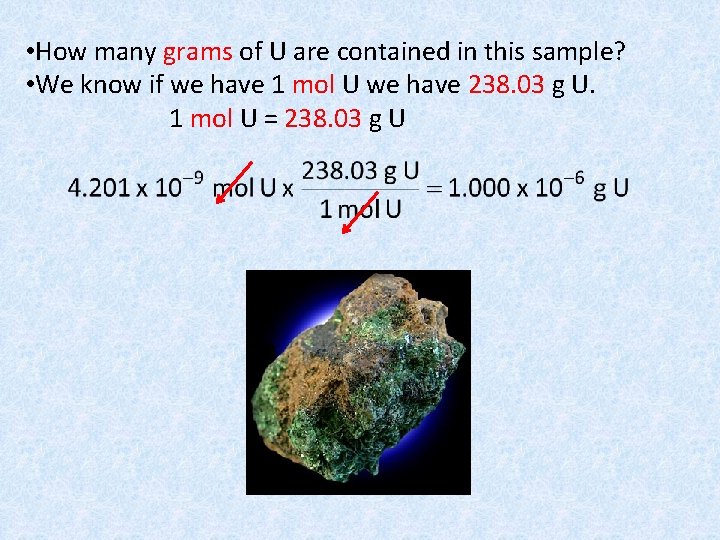
• How many grams of U are contained in this sample? • We know if we have 1 mol U we have 238. 03 g U. 1 mol U = 238. 03 g U

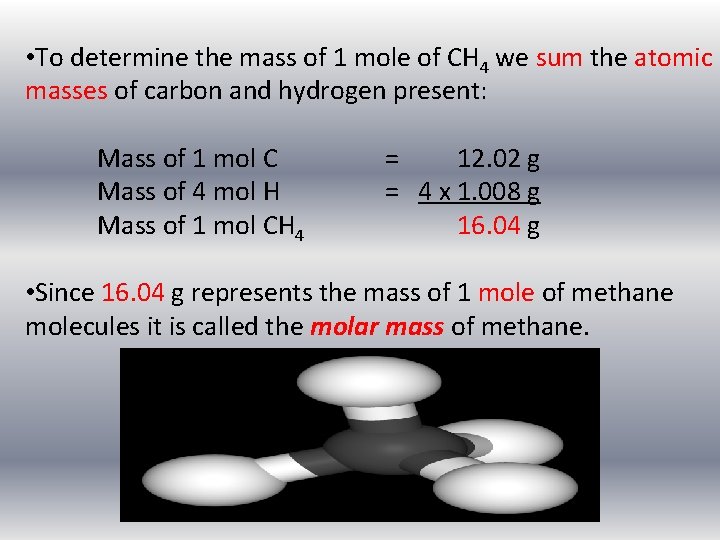
• Molar mass of a substance is the mass in grams of one mole of the compound. • Molar mass has units of g/mol. • Remember a chemical compound is a collection of bonded atoms. • If we want to know the mass in grams of one mole of methane, CH 4 (the mass of 6. 02 x 1023 atoms of CH 4) we need to take into account that 1 mole of CH 4 contains 1 mole of carbon atoms and 4 moles of hydrogen atoms.

• To determine the mass of 1 mole of CH 4 we sum the atomic masses of carbon and hydrogen present: Mass of 1 mol C Mass of 4 mol H Mass of 1 mol CH 4 = 12. 02 g = 4 x 1. 008 g 16. 04 g • Since 16. 04 g represents the mass of 1 mole of methane molecules it is called the molar mass of methane.

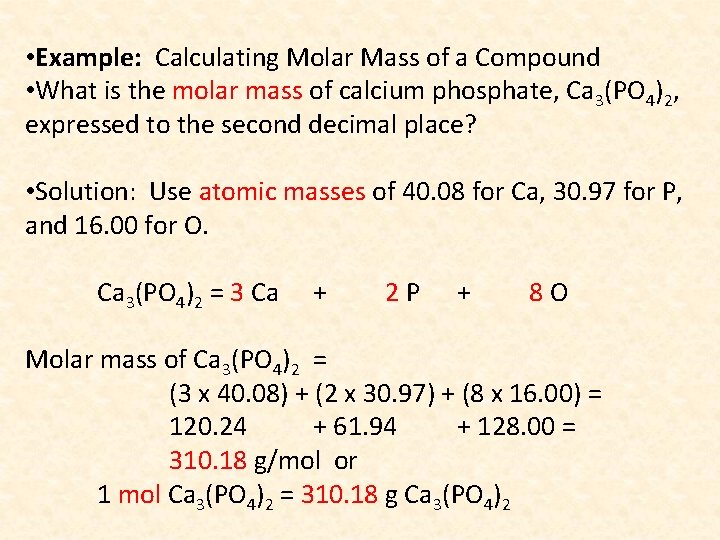
• Example: Calculating Molar Mass of a Compound • What is the molar mass of calcium phosphate, Ca 3(PO 4)2, expressed to the second decimal place? • Solution: Use atomic masses of 40. 08 for Ca, 30. 97 for P, and 16. 00 for O. Ca 3(PO 4)2 = 3 Ca + 2 P + 8 O Molar mass of Ca 3(PO 4)2 = (3 x 40. 08) + (2 x 30. 97) + (8 x 16. 00) = 120. 24 + 61. 94 + 128. 00 = 310. 18 g/mol or 1 mol Ca 3(PO 4)2 = 310. 18 g Ca 3(PO 4)2

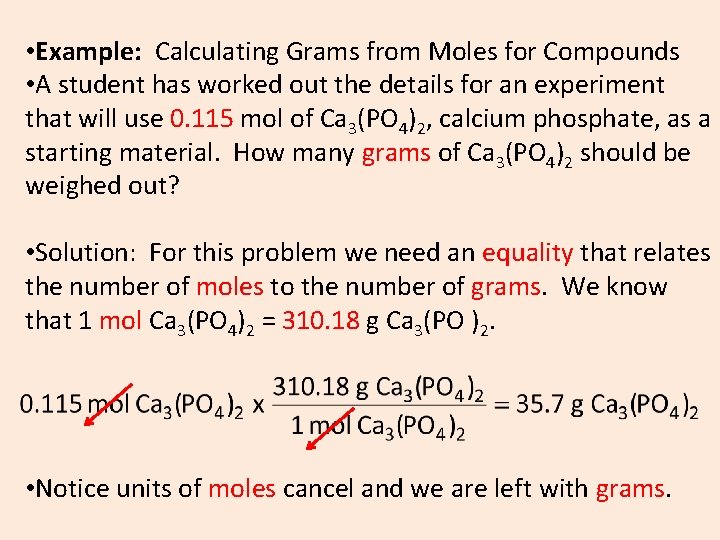
• Example: Calculating Grams from Moles for Compounds • A student has worked out the details for an experiment that will use 0. 115 mol of Ca 3(PO 4)2, calcium phosphate, as a starting material. How many grams of Ca 3(PO 4)2 should be weighed out? • Solution: For this problem we need an equality that relates the number of moles to the number of grams. We know that 1 mol Ca 3(PO 4)2 = 310. 18 g Ca 3(PO )2. • Notice units of moles cancel and we are left with grams.

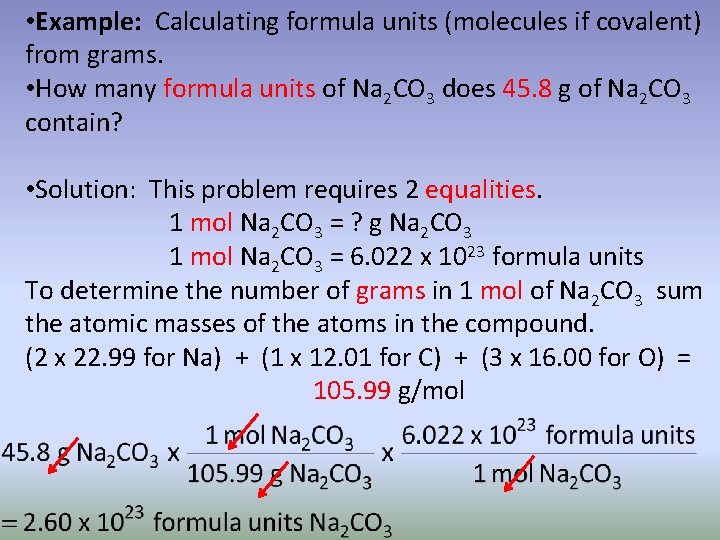
• Example: Calculating formula units (molecules if covalent) from grams. • How many formula units of Na 2 CO 3 does 45. 8 g of Na 2 CO 3 contain? • Solution: This problem requires 2 equalities. 1 mol Na 2 CO 3 = ? g Na 2 CO 3 1 mol Na 2 CO 3 = 6. 022 x 1023 formula units To determine the number of grams in 1 mol of Na 2 CO 3 sum the atomic masses of the atoms in the compound. (2 x 22. 99 for Na) + (1 x 12. 01 for C) + (3 x 16. 00 for O) = 105. 99 g/mol

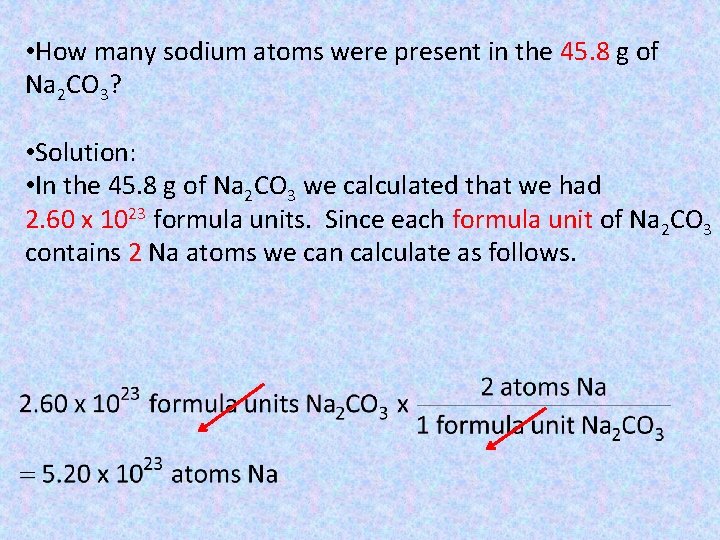
• How many sodium atoms were present in the 45. 8 g of Na 2 CO 3? • Solution: • In the 45. 8 g of Na 2 CO 3 we calculated that we had 2. 60 x 1023 formula units. Since each formula unit of Na 2 CO 3 contains 2 Na atoms we can calculate as follows.