The Mole The Basic Chemical Unit A Mole

The Mole The Basic Chemical Unit

A Mole is : • A chemical quantity • A Mole of any substance is Chemically equivalent to a mole of any other substance

A Mole is : Anything that has the same number of particles as the number of atoms in exactly 12. 0 grams of Carbon-12.

Avogadro’s Number 23 6. 02 x 10
Representative Particles HOW TO COUNT

Elements Atoms
Covalent Compounds Molecules

Ionic Compounds Formula Units

One Mole of : • Carbon = 6. 02 x Carbon Atoms 23 • Silicon = 6. 02 x 10 Silicon atoms 23 10

One Mole of : • CO 2 = 6. 02 x Carbon Di. Oxide Molecules 23 • HNO 3 = 6. 02 x 10 Nitric Acid Molecules 23 10

One Mole of : • Platinum = _______ Platinum Atoms • Scandium = ______ Scandium atoms
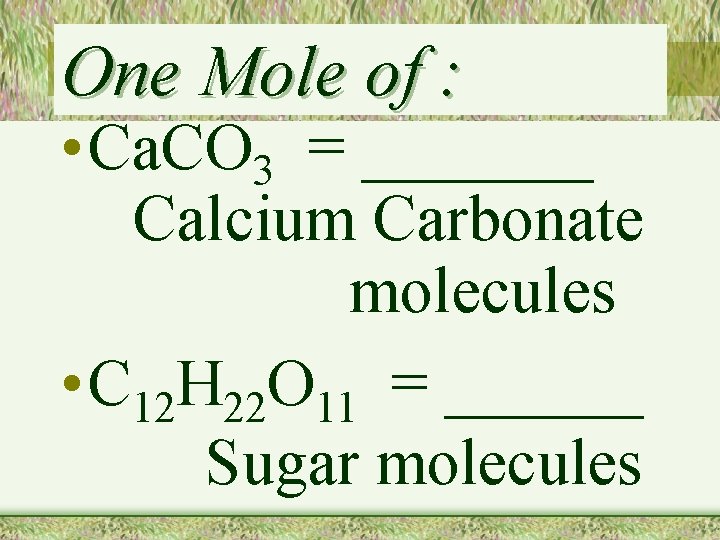
One Mole of : • Ca. CO 3 = _______ Calcium Carbonate molecules • C 12 H 22 O 11 = ______ Sugar molecules

Molar Mass

Defination The mass of one mole of a substance.

One Mole of : • Carbon = 12. 0 g • Silicon = 28. 1 g • Barium = 137. 3 g • Helium = 4. 0 g
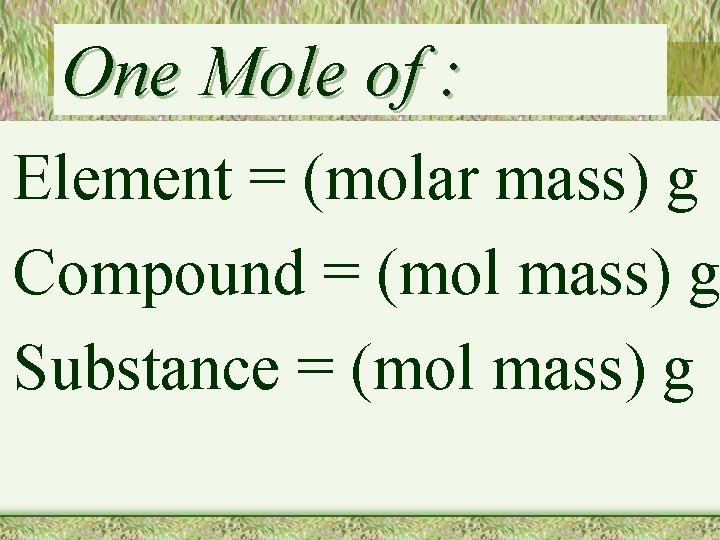
One Mole of : Element = (molar mass) g Compound = (mol mass) g Substance = (mol mass) g

One Mole of : • Aluminum = ____ g • Arsenic = ____ g • Silver = _____ g • Xenon = _____ g

One Mole of : • CO 2 = 44. 0 g • SO 3 = 80. 1 g • Ba. SO 4 = 233. 4 g • (NH 4)3 PO 4 = 149. 0 g
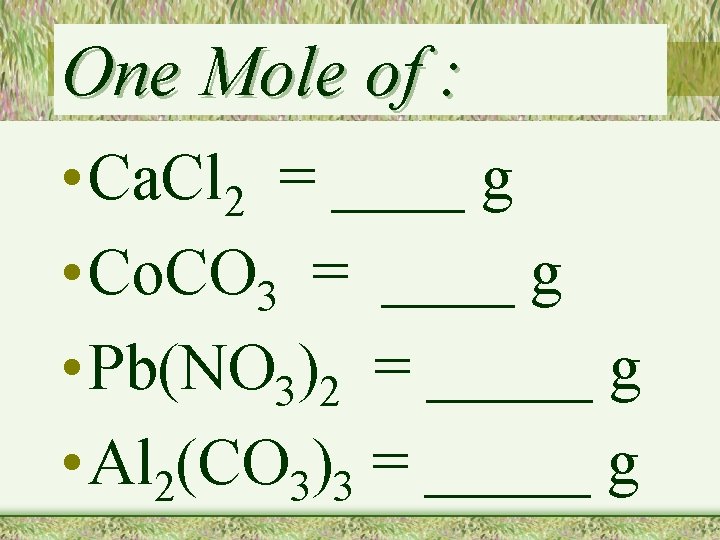
One Mole of : • Ca. Cl 2 = ____ g • Co. CO 3 = ____ g • Pb(NO 3)2 = _____ g • Al 2(CO 3)3 = _____ g
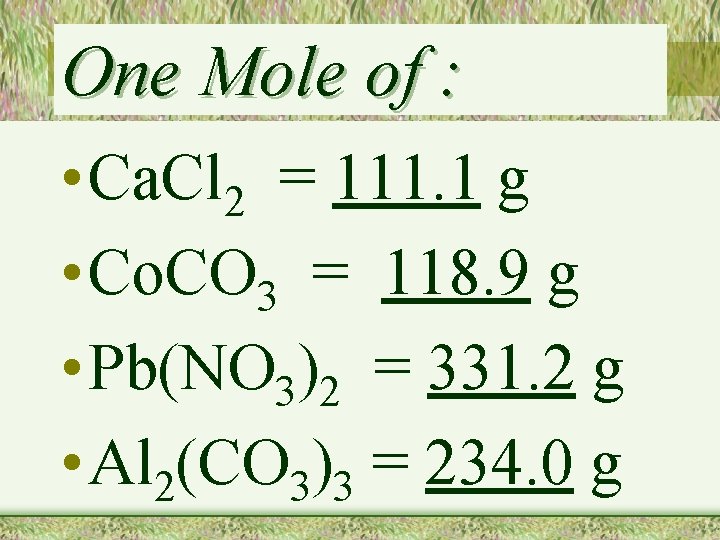
One Mole of : • Ca. Cl 2 = 111. 1 g • Co. CO 3 = 118. 9 g • Pb(NO 3)2 = 331. 2 g • Al 2(CO 3)3 = 234. 0 g

Molar Volume

Molar Volume The volume of one mole of gas @STP.

STP Standard Temperature and Pressure

1 mole of gas @ STP 22. 4 liters

Does not matter what the gas is.
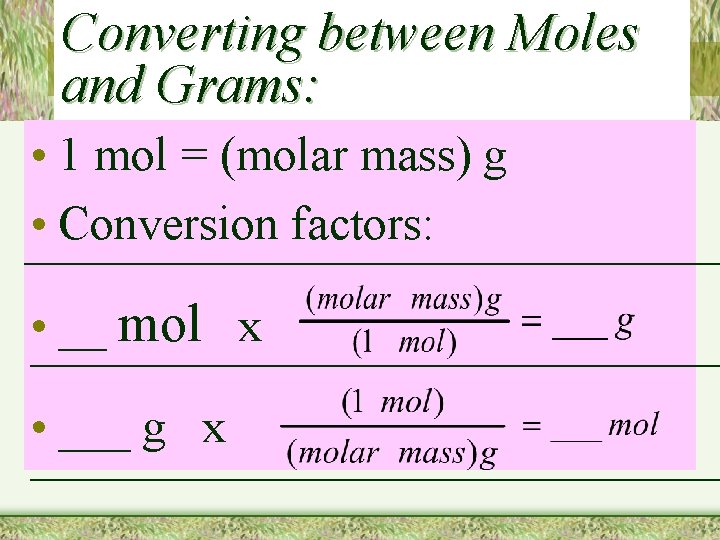
Converting between Moles and Grams: • 1 mol = (molar mass) g • Conversion factors: • __ mol x • ___ g x
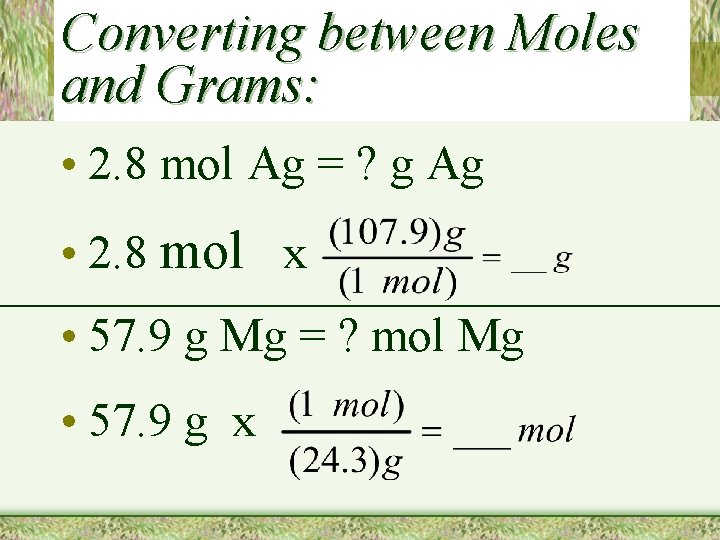
Converting between Moles and Grams: • 2. 8 mol Ag = ? g Ag • 2. 8 mol x • 57. 9 g Mg = ? mol Mg • 57. 9 g x
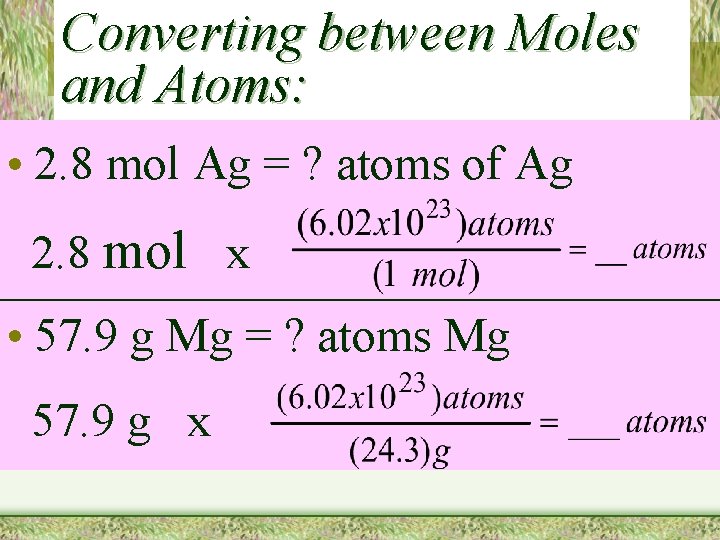
Converting between Moles and Atoms: • 2. 8 mol Ag = ? atoms of Ag 2. 8 mol x • 57. 9 g Mg = ? atoms Mg 57. 9 g x
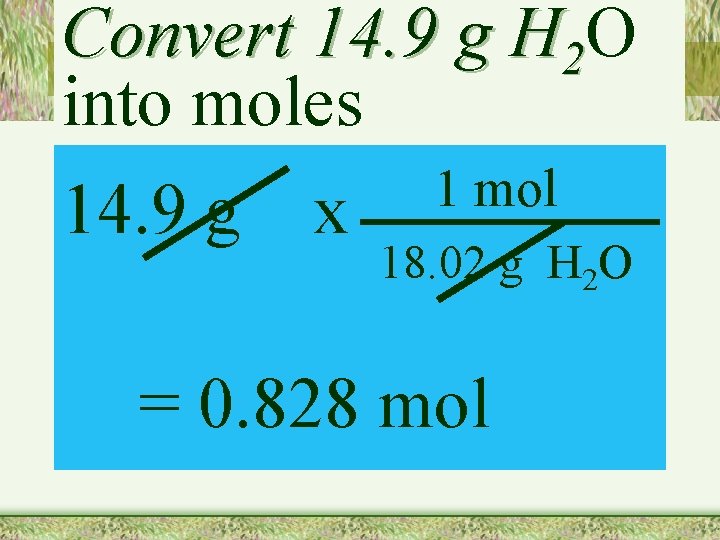
Convert 14. 9 g H 2 O into moles 14. 9 g x 1 mol 18. 02 g H 2 O = 0. 828 mol
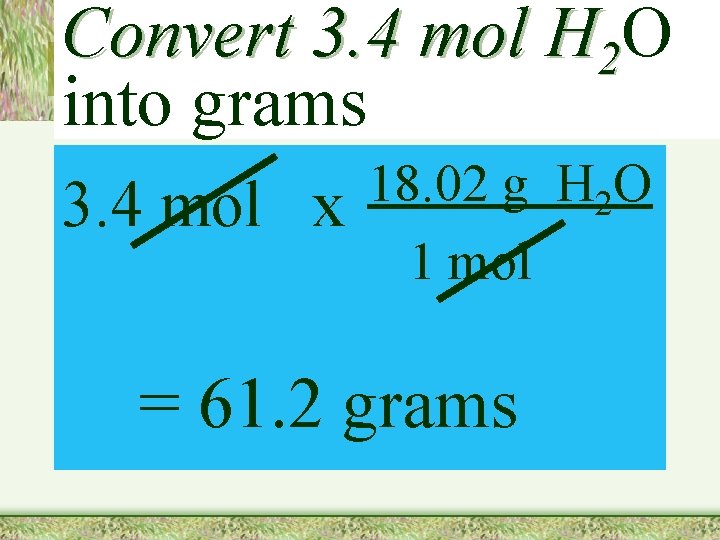
Convert 3. 4 mol H 2 O into grams 3. 4 mol x 18. 02 g H 2 O 1 mol = 61. 2 grams

Convert 25. 1 g of CO 2 into moles: • 25. 1 g x 1 mol CO 2 44. 01 g CO 2 = 0. 570 mol
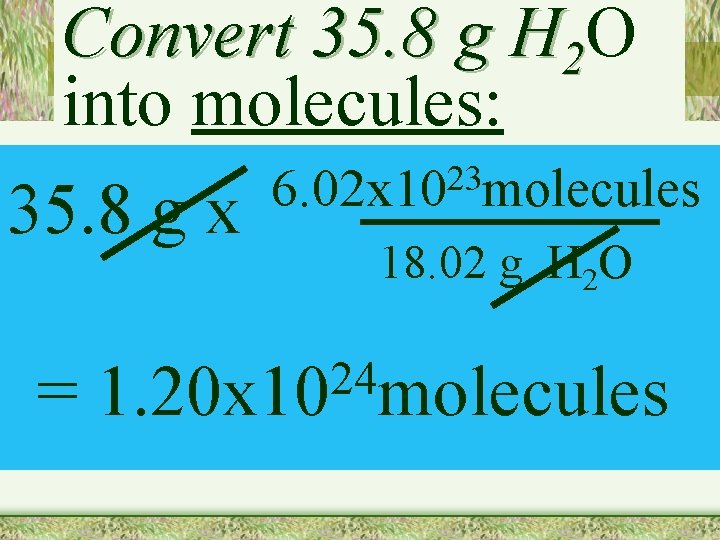
Convert 35. 8 g H 2 O into molecules: 35. 8 g x = 23 6. 02 x 10 molecules 18. 02 g H 2 O 24 1. 20 x 10 molecules
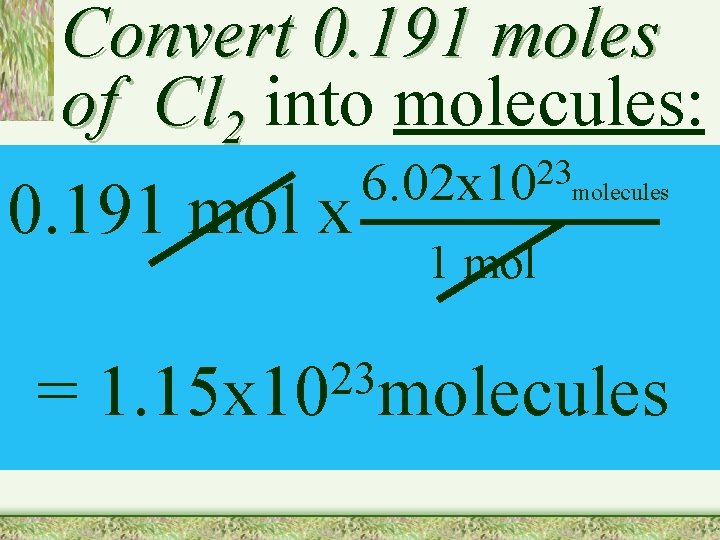
Convert 0. 191 moles of Cl 2 into molecules: 0. 191 mol x = 23 6. 02 x 10 molecules 1 mol 23 1. 15 x 10 molecules
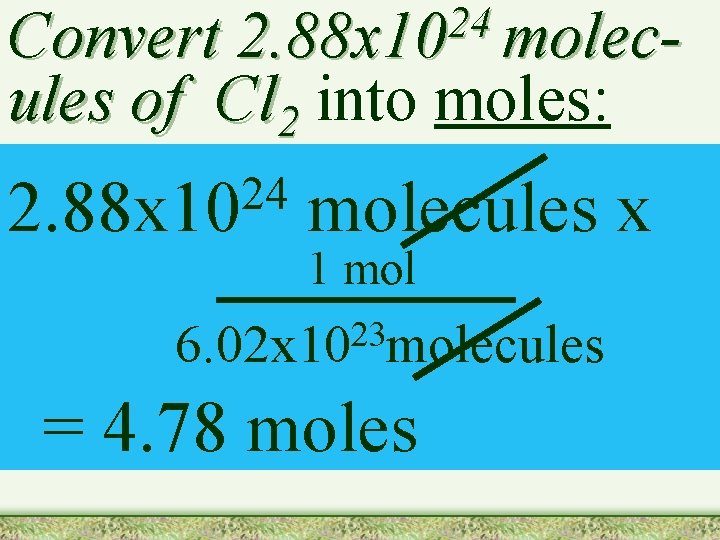
24 2. 88 x 10 molec- Convert ules of Cl 2 into moles: 24 2. 88 x 10 molecules x 1 mol 23 6. 02 x 10 molecules = 4. 78 moles
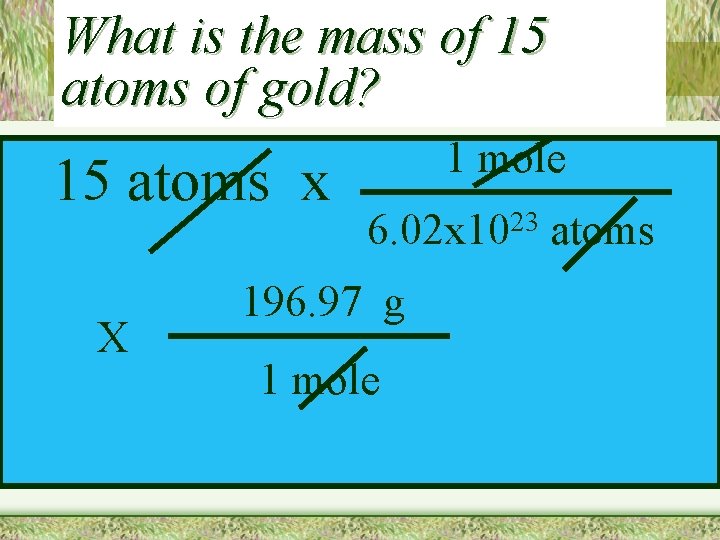
What is the mass of 15 atoms of gold? 15 atoms x X 1 mole 23 6. 02 x 10 196. 97 g 1 mole atoms
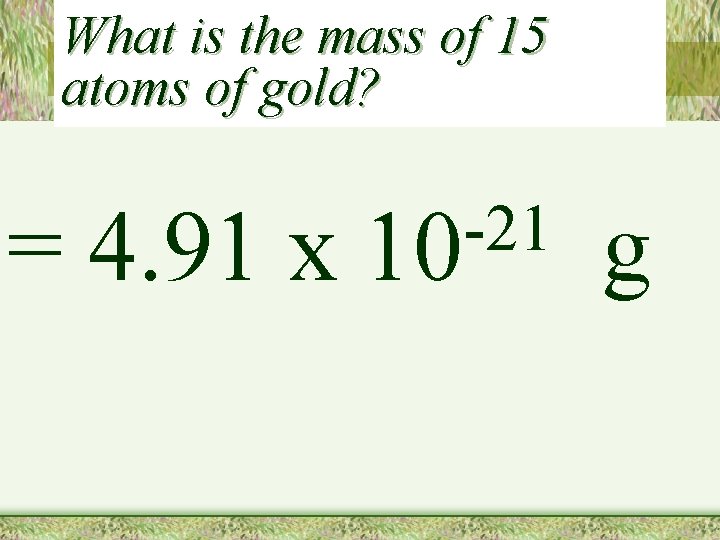
What is the mass of 15 atoms of gold? = 4. 91 x -21 10 g
- Slides: 36