The Mole Remembering Terms Atomic number of Mass

The Mole
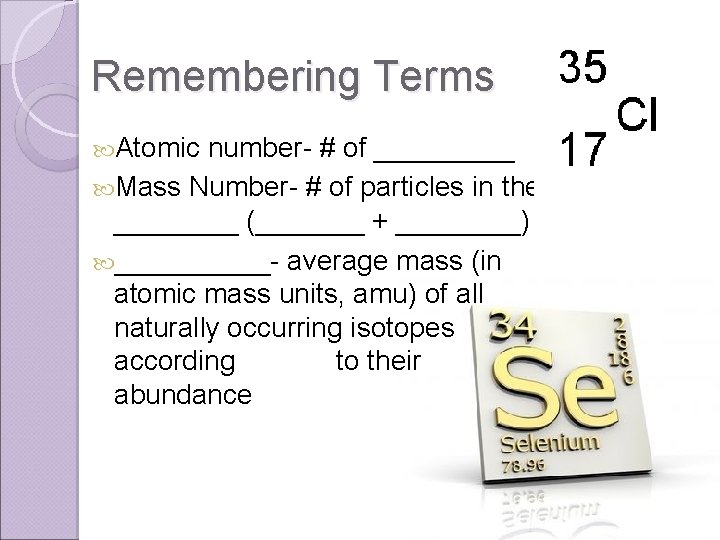
Remembering Terms Atomic number- # of _____ Mass Number- # of particles in the ____ (_______ + ____) _____- average mass (in atomic mass units, amu) of all naturally occurring isotopes according to their abundance

Given: one atom 56 Fe ◦ What is the atomic #? _____ ◦ What is the mass #? ______ ◦ What is the atomic mass? ______ Given: a sample of iron ◦ What is the atomic #? _______ ◦ What is the mass #? _______ ◦ What is the atomic mass? _______
Calculating Atomic Mass Finding atomic mass of an element ◦ Look it up on the periodic table ◦ What is the atomic mass of Na? C? Ag? Na C Ag ________ Finding atomic mass of a compound ◦ Calculate it using each element’s atomic mass from the periodic table ◦ Remember to take into account how many atoms of each type are in the compound ◦ What is the atomic mass of Na. Cl? H 2 O? Na 3 PO 4? Na. Cl H 2 O Na 3 PO 4 ________

The Mole A name for a certain number of things ◦ Just like A dozen is the name for ___ things A baker’s dozen is the name for ___ things A gross is the name for ____ things (a dozens) A mole(mol) is the name for ____things, also known as Avogadro’s number ◦ Atoms, molecules, formula units
Using Avogadro’s Number Calculating number of moles of each element in a compound ◦ How many moles of Na are in 1 mol of Na. Cl? _______________ ◦ How many moles of Cl are in 3 moles of Mg. Cl 2? _______________ Calculating number of things ◦ How many molecules are in 4 moles of CO 2? ◦ How many atoms are in 2. 5 moles of CO 2?
The Mole (cont) Definition of the Mole ◦ A mole equals the number of atoms in 12 g of carbon-12 Because of this definition, ◦ Atomic mass in amu’s equals the mass in grams (molecular mass or molar mass) ◦ What is the molecular mass of Na? C? Ag? Na – ______ C – _______ Ag – ______ ◦ What is the molecular mass of Na. Cl? H 2 O? Na 3 PO 4? Na. Cl – ______
Molar Calculations Using Mass Calculating # of moles from mass or mass from number of moles ◦ How many moles of Na. NO 3 are in 308. 25 g? How many moles of O are in the sample? ◦ How many kilograms are in 28. 34 moles of Na. Cl?
Molar Calculations Using Mass (cont) Calculating # of things from mass or mass from number of things ◦ How many molecules of water are in 20 g? ◦ How many atoms are in 0. 37 g of N 2?
% Composition of Compounds Gives the % of each element in a compound by mass (Molecular mass of element in compound/ Molecular mass of compound) x 100 What is the % composition of each element in Na 2 SO 3? ◦ Na ____________ ◦S ___________
Empirical Formulas Simplest, reduced formula for a compound In (almost all) ionic compounds, the empirical formula matches the molecular formula ◦ For example: Magnesium chloride Molecular formula- ______ Empirical formula- _______ In covalent compound, they often don’t match ◦ For example: Glucose Molecular formula- ______ Empirical formula- _______
Calculating Empirical Formulas Known: ◦ % of compound that each element represents (% composition) or ◦ how many grams of each element are in the compound Steps 1. Change each element’s amount in g to amount in mol If given % comp, the number of grams is the %.

Calculating Empirical Formulas Steps (cont) 2. Find the smallest amount of moles among the elements and divide each number of moles by this number 3. Determine the empirical formula If the divided numbers are whole integers (or very close), then these are the subscripts for each of the corresponding elements. If some of the divided numbers are not whole integers, multiply all of them by a # that will make all of them whole integers
Calculating Empirical Formulas What is the empirical formula of a compound that is 76. 57% C, 6. 43% H, and 17% O by mass? Step 1 Step 2 Step 3
Calculating Empirical Formulas What is the empirical formula for a compound that contains C, H, and O if a 20 g sample has 9. 054 g C and 1. 900 g H? Step 1 Step 2 Step 3
Calculating Empirical Formula What is the formula for a hydrate if the compound contains 79. 07% Ca. SO 4 and 20. 93% H 20? Step 1 Step 2 Step 3
Calculating Molecular Formulas Known: ◦ Empirical formula (and therefore empirical formula mass) ◦ Molecular mass Steps 1. Calculate empirical formula mass (same as calculating a molecular mass but use empirical formula) 2. Divide molecular mass by empirical formula mass to get a whole integer 3. Multiple all elements in the empirical formula by the whole integer to get the molecular formula.

Calculating Molecular Formulas If the empirical formula of hydroquinone is C 3 H 3 O and the molecular mass is 110 g/mol, what is the molecular formula? Step 1 Step 2 Step 3
- Slides: 18