The Mole Ratio MYP G 10 Science BCIS

The Mole Ratio MYP G 10 Science BCIS Matthew Ignash

Learning Objectives: • I can determine the mole conversion from a balanced chemical reaction. • I can convert from grams, liters, atoms, etc. from a reactant to a product.

Bell Ringer: January 7, 2019 • Complete the bell ringer sheet ”Seedlings in a Jar” • Think, Pair, Share (Individual, Partner, Class Discussion
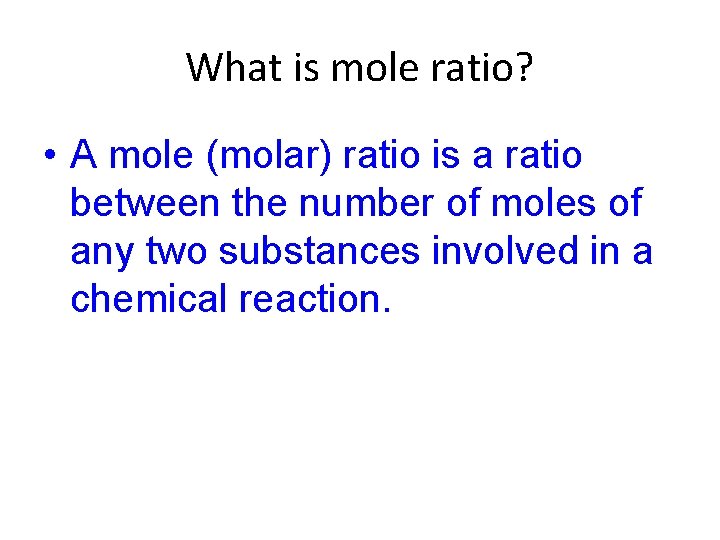
What is mole ratio? • A mole (molar) ratio is a ratio between the number of moles of any two substances involved in a chemical reaction.

Stoichiometry

Activity Using modeling kits: • Make a model of 2 hydrogen and 1 oxygen molecules • Using the above parts to make 2 water molecules. How this models a chemical reaction?
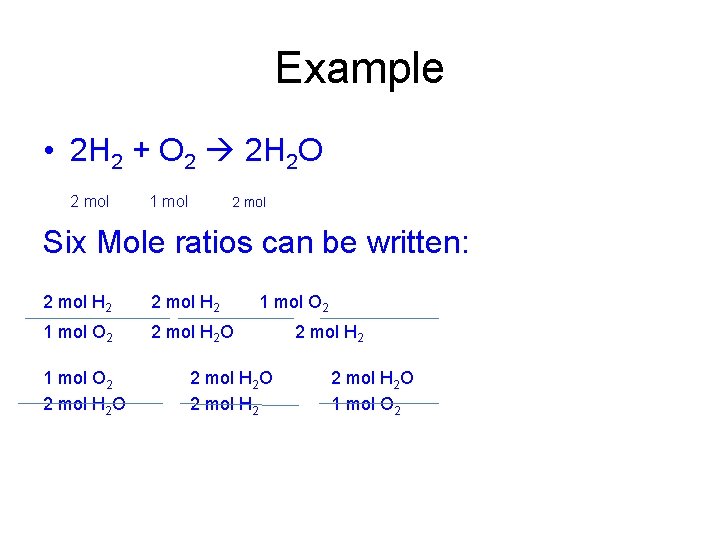
Example • 2 H 2 + O 2 2 H 2 O 2 mol 1 mol 2 mol Six Mole ratios can be written: 2 mol H 2 1 mol O 2 2 mol H 2 O 2 mol H 2 O 1 mol O 2

Why mole ratio is important? • We use the mole ratio to convert the number of moles of one substance to the corresponding number of moles of another substance in a chemical reaction.
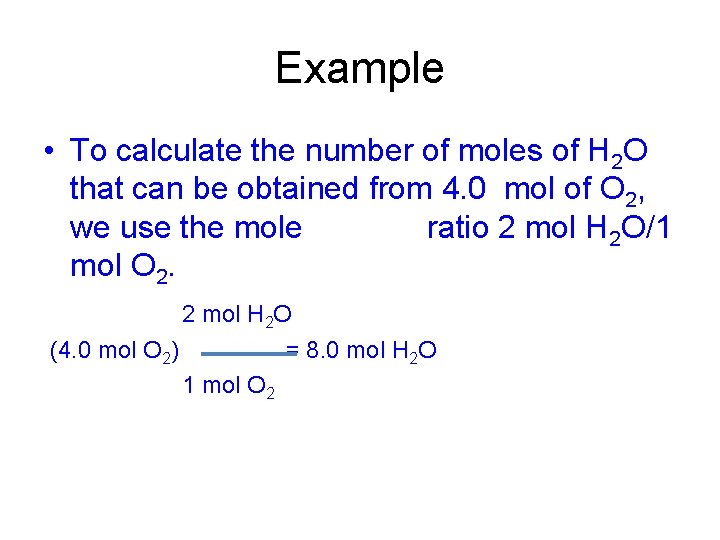
Example • To calculate the number of moles of H 2 O that can be obtained from 4. 0 mol of O 2, we use the mole ratio 2 mol H 2 O/1 mol O 2. 2 mol H 2 O (4. 0 mol O 2) = 8. 0 mol H 2 O 1 mol O 2
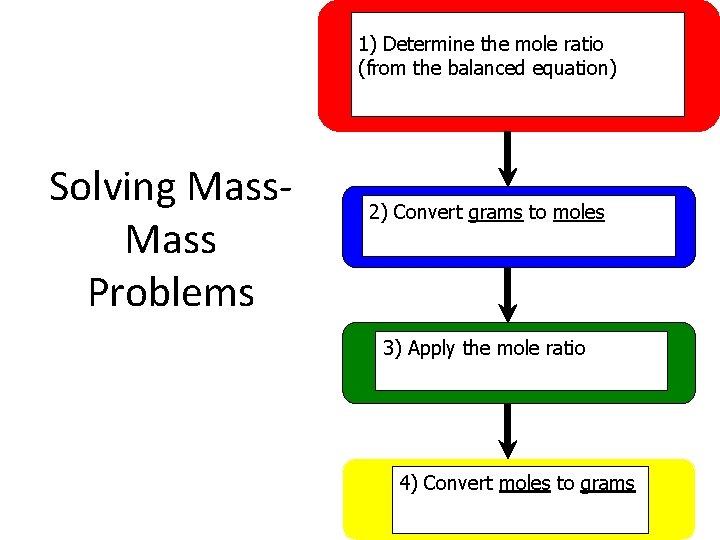
1) Determine the mole ratio (from the balanced equation) Solving Mass Problems 2) Convert grams to moles 3) Apply the mole ratio 4) Convert moles to grams
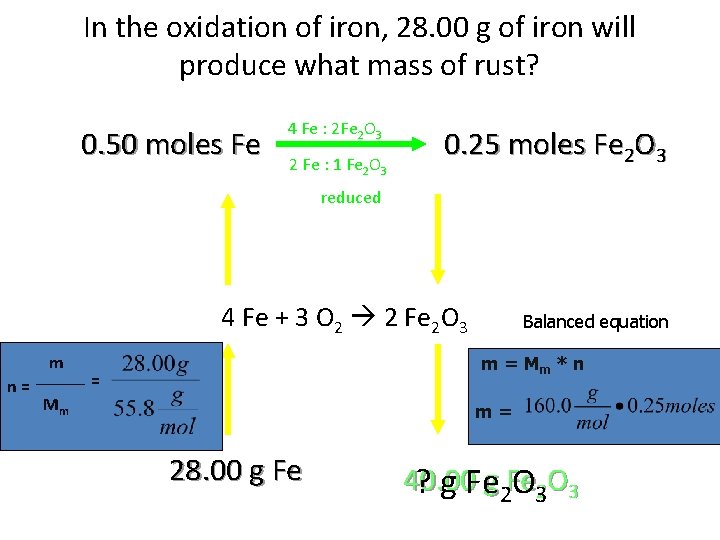
In the oxidation of iron, 28. 00 g of iron will produce what mass of rust? 0. 50 moles Fe 4 Fe : 2 Fe 2 O 3 2 Fe : 1 Fe 2 O 3 0. 25 moles Fe 2 O 3 reduced 4 Fe + 3 O 2 2 Fe 2 O 3 m n= Balanced equation m = Mm * n = Mm m= 28. 00 g Fe 40. 00 ? g Feg 2 Fe O 32 O 3
- Slides: 11