The Mole mole mol number of particles equal

The Mole • mole (mol) = number of particles equal to the number of atoms in 12 g of C-12 üone mole of anything is 6. 022 x 1023 units of that thing üwhere 6. 022 x 1023 is known as NA Avogadro’s Number(often denoted ___) _________ ü 1 mol of marbles = 6. 022 x 1023 marbles x 1023 He atoms ü 1 mol of He = 6. 022 _____ ü 1 mol of CO 2 = _____ 6. 022 x 1023 CO 2 molecules

Converting Between Moles and Number of Atoms Example: • A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? = 1. 8266 x 10 -2 moles Ag • Sig. Figs. & Round: = 1. 8 x 10 -2 moles Ag

Relationship Between Moles and Mass • The mass of one mole of atoms is called the molar mass _____ • The molar mass of an element, in grams, is numerically equal to the element’s atomic mass, in amu ü atomic mass of Cu = 63. 55 _____ g/mol ü molar mass of Cu = 63. 55 ______
Mole and Mass Relationships 1 mole Sulfur 32. 06 g 1 mole Carbon 12. 01 g
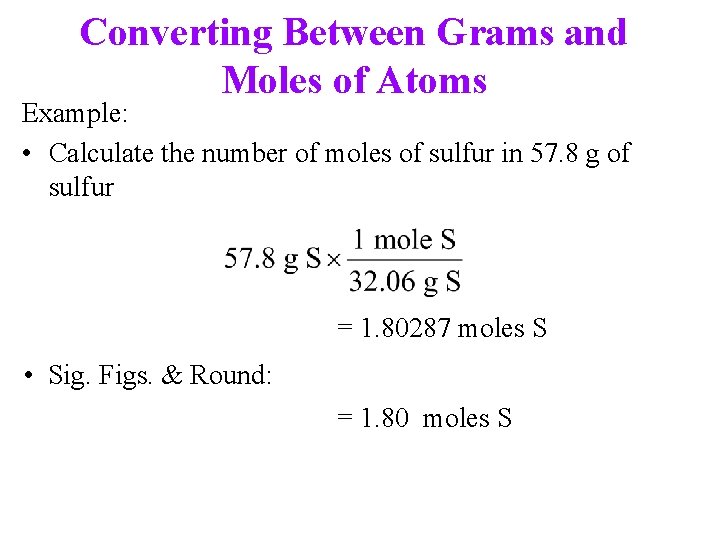
Converting Between Grams and Moles of Atoms Example: • Calculate the number of moles of sulfur in 57. 8 g of sulfur = 1. 80287 moles S • Sig. Figs. & Round: = 1. 80 moles S

Converting Between Grams and Number of Atoms Example: • How many aluminum atoms are in an aluminum can with a mass of 16. 2 g? = 3. 6159 x 1023 atoms Al • Sig. Figs. & Round: = 3. 62 x 1023 atoms Al
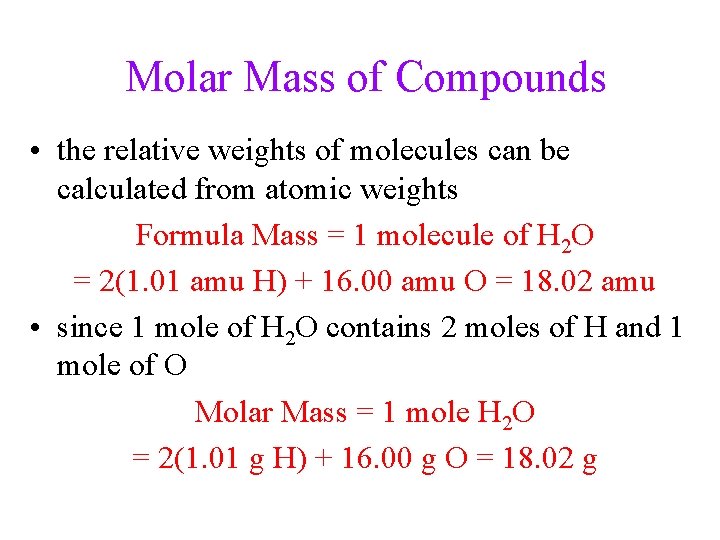
Molar Mass of Compounds • the relative weights of molecules can be calculated from atomic weights Formula Mass = 1 molecule of H 2 O = 2(1. 01 amu H) + 16. 00 amu O = 18. 02 amu • since 1 mole of H 2 O contains 2 moles of H and 1 mole of O Molar Mass = 1 mole H 2 O = 2(1. 01 g H) + 16. 00 g O = 18. 02 g

Converting Between Grams and Number of Molecules Example: • Find the mass of 4. 78 x 1024 NO 2 molecules? = 365. 21 g NO 2 • Sig. Figs. & Round: = 365 g NO 2
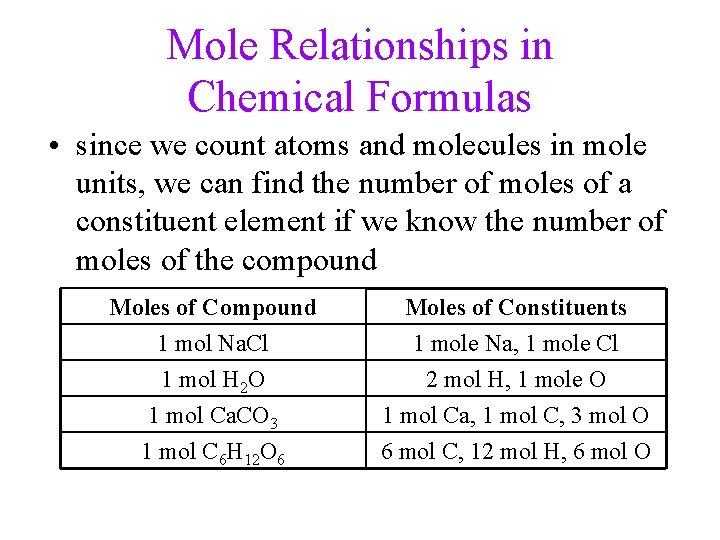
Mole Relationships in Chemical Formulas • since we count atoms and molecules in mole units, we can find the number of moles of a constituent element if we know the number of moles of the compound Moles of Compound 1 mol Na. Cl 1 mol H 2 O 1 mol Ca. CO 3 Moles of Constituents 1 mole Na, 1 mole Cl 2 mol H, 1 mole O 1 mol Ca, 1 mol C, 3 mol O 1 mol C 6 H 12 O 6 6 mol C, 12 mol H, 6 mol O
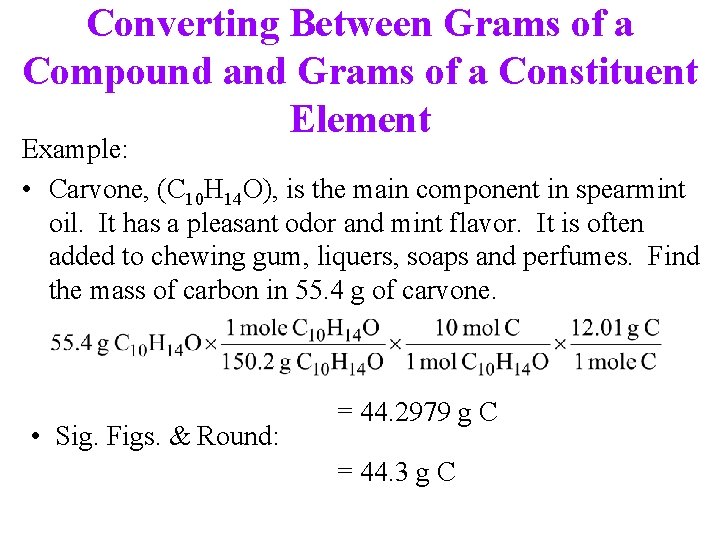
Converting Between Grams of a Compound and Grams of a Constituent Element Example: • Carvone, (C 10 H 14 O), is the main component in spearmint oil. It has a pleasant odor and mint flavor. It is often added to chewing gum, liquers, soaps and perfumes. Find the mass of carbon in 55. 4 g of carvone. • Sig. Figs. & Round: = 44. 2979 g C = 44. 3 g C
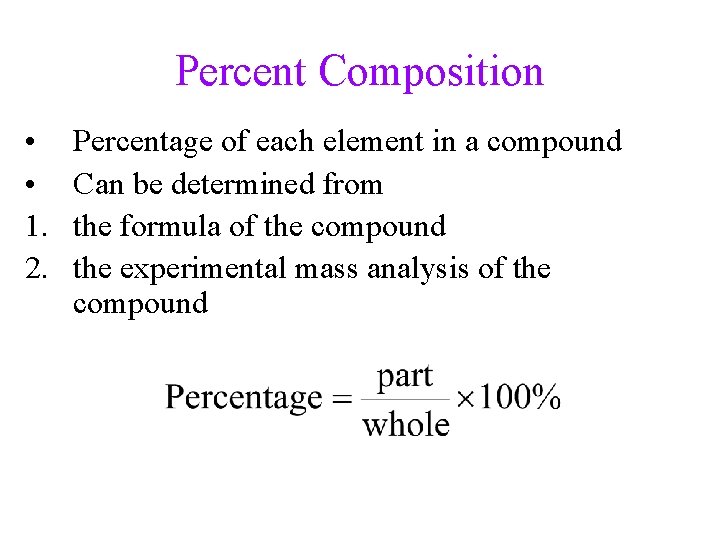
Percent Composition • • 1. 2. Percentage of each element in a compound Can be determined from the formula of the compound the experimental mass analysis of the compound

Example - Percent Composition from the Formula C 2 H 5 OH 1. Determine the mass of each element in 1 mole of the compound 2 moles C = 2(12. 01 g) = 24. 02 g 6 moles H = 6(1. 008 g) = 6. 048 g 1 mol O = 1(16. 00 g) = 16. 00 g 2. Determine the molar mass of the compound by adding the masses of the elements 1 mole C 2 H 5 OH = 46. 07 g

Example - Percent Composition from the Formula C 2 H 5 OH 3. Divide the mass of each element by the molar mass of the compound and multiply by 100%

Empirical Formulas • The simplest, whole-number ratio of atoms in a Formula molecule is called the Empirical __________ ücan be determined from percent composition or combining masses • The Molecular Formula is a multiple of the Empirical Formula Glucose Molecular Formula = C 6 H 12 O 6 Empirical Formula = CH 2 O

1) 2) 3) 4) 5) Finding an Empirical Formula convert the percentages to grams a) skip if already grams convert grams to moles a) use molar mass of each element write a pseudoformula using moles as subscripts divide all by smallest number of moles multiply all mole ratios by a small whole number to make all whole number subscripts fractional subscript multiply by. 10 10 N 1 O 2. 5 x 2 N 2 O 5. 20 5. 25 4. 33 3. 50 2. 66 3. 75 4
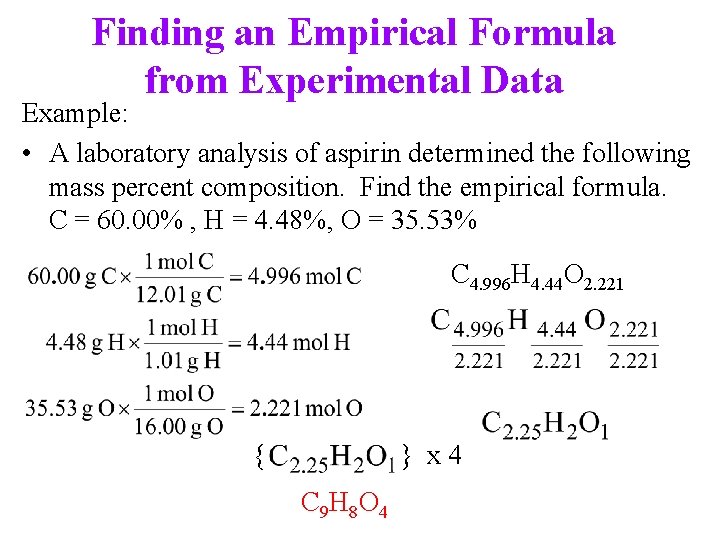
Finding an Empirical Formula from Experimental Data Example: • A laboratory analysis of aspirin determined the following mass percent composition. Find the empirical formula. C = 60. 00% , H = 4. 48%, O = 35. 53% C 4. 996 H 4. 44 O 2. 221 { } x 4 C 9 H 8 O 4
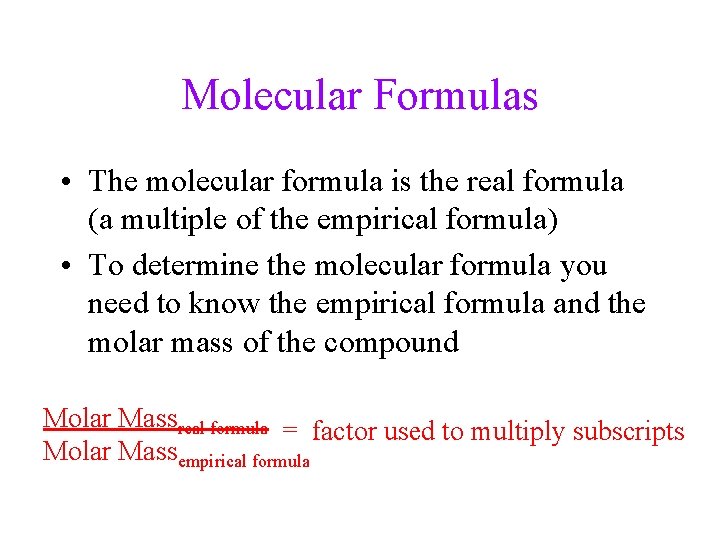
Molecular Formulas • The molecular formula is the real formula (a multiple of the empirical formula) • To determine the molecular formula you need to know the empirical formula and the molar mass of the compound Molar Massreal formula = factor used to multiply subscripts Molar Massempirical formula
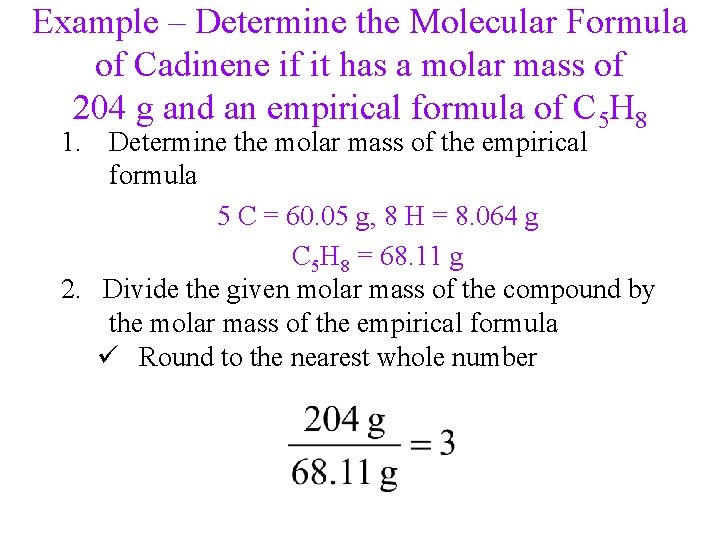
Example – Determine the Molecular Formula of Cadinene if it has a molar mass of 204 g and an empirical formula of C 5 H 8 1. Determine the molar mass of the empirical formula 5 C = 60. 05 g, 8 H = 8. 064 g C 5 H 8 = 68. 11 g 2. Divide the given molar mass of the compound by the molar mass of the empirical formula ü Round to the nearest whole number

Example – Determine the Molecular Formula of Cadinene if it has a molar mass of 204 g and an empirical formula of C 5 H 8 3. Multiply the empirical formula by the factor above to give the molecular formula (C 5 H 8)3 = C 15 H 24
- Slides: 19