The Mole is Back Ions Formula Units Mrs
The Mole is Back Ions & Formula Units Mrs. Page General Chemistry 2015 -2016

Learning Objectives • Calculate the number of molecules, formula units, or ions in a given molar amount of a chemical compound • Use dimensional analysis to perform calculations • Perform mathematical calculations involving significant figures.
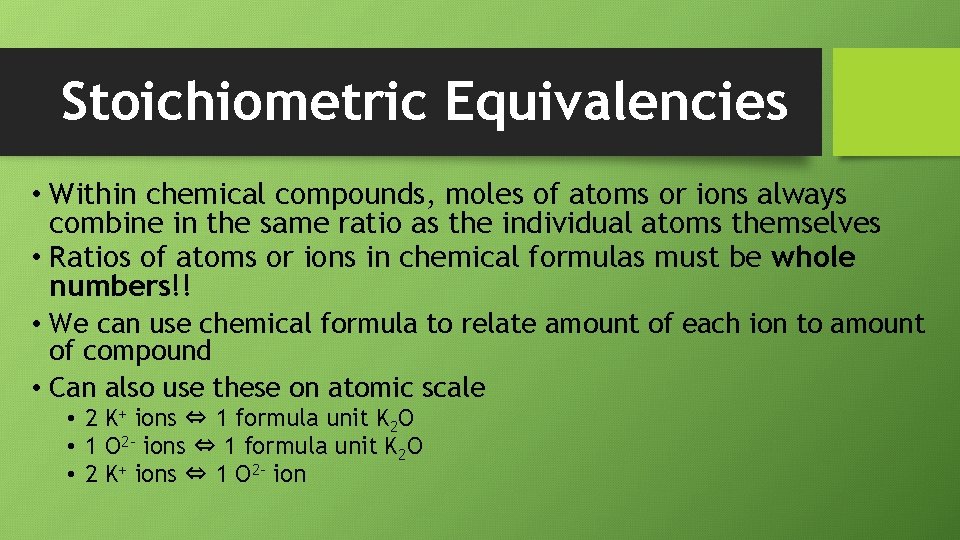
Stoichiometric Equivalencies • Within chemical compounds, moles of atoms or ions always combine in the same ratio as the individual atoms themselves • Ratios of atoms or ions in chemical formulas must be whole numbers!! • We can use chemical formula to relate amount of each ion to amount of compound • Can also use these on atomic scale • 2 K+ ions ⇔ 1 formula unit K 2 O • 1 O 2 - ions ⇔ 1 formula unit K 2 O • 2 K+ ions ⇔ 1 O 2 - ion
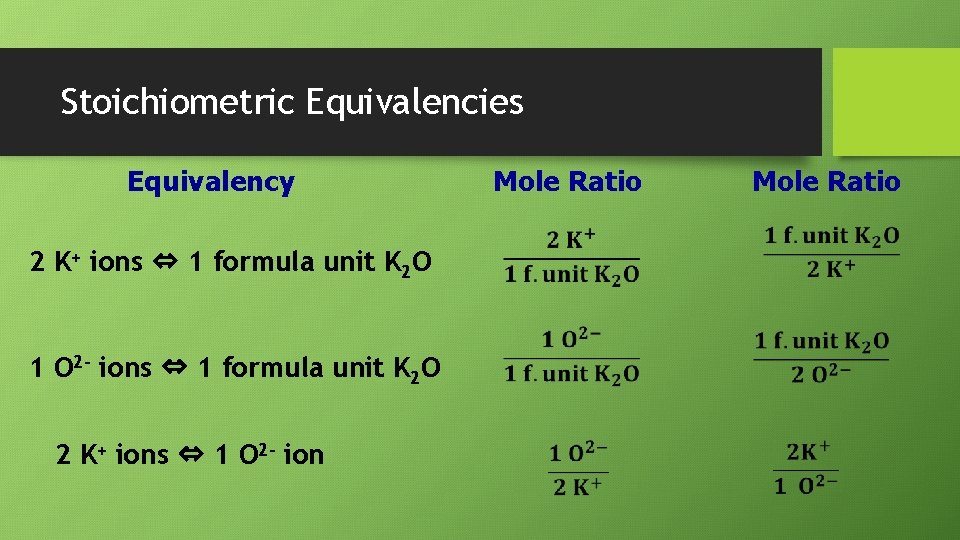
Stoichiometric Equivalencies Equivalency 2 K+ ions ⇔ 1 formula unit K 2 O 1 O 2 - ions ⇔ 1 formula unit K 2 O 2 K+ ions ⇔ 1 O 2 - ion Mole Ratio

Dimensional Analysis Steps (REVIEW) • Start with known quantity. • Use conversion factors (stoichiometric equivalents) – set up so that units cancel • Continue until you are to the unit desired • Perform calculation • Round to appropriate Significant Figures • Write final answer with units!

Significant Figures Review (REVIEW) • When adding and subtracting – use least number of decimal places • When multiplying and dividing – use smallest number of significant figures. • How do you know if a number is significant? (SEE PPT from Unit 1)
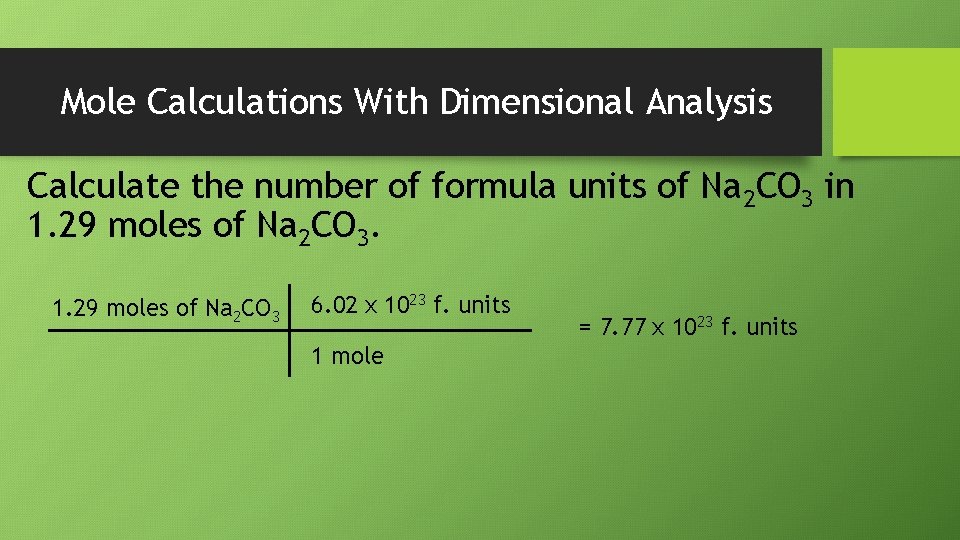
Mole Calculations With Dimensional Analysis Calculate the number of formula units of Na 2 CO 3 in 1. 29 moles of Na 2 CO 3 6. 02 x 1023 f. units 1 mole = 7. 77 x 1023 f. units
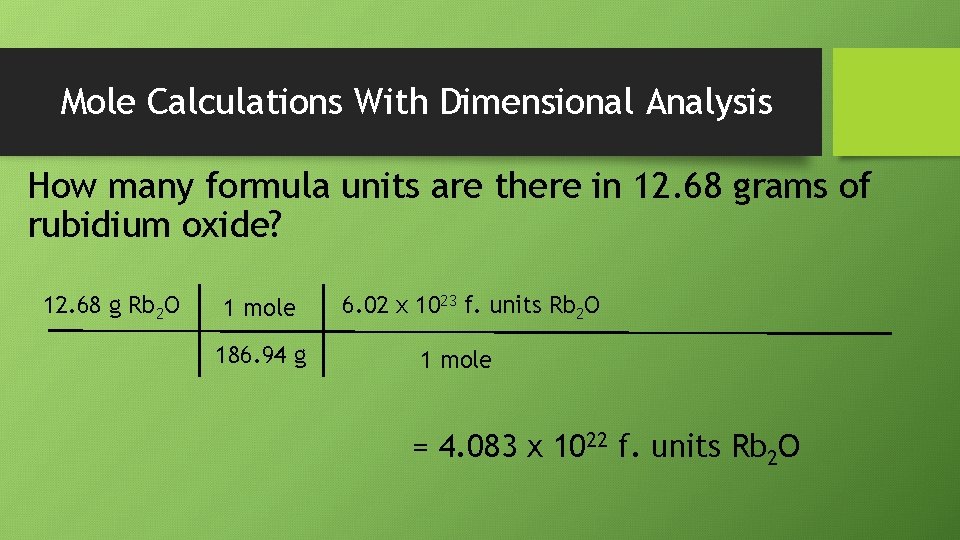
Mole Calculations With Dimensional Analysis How many formula units are there in 12. 68 grams of rubidium oxide? 12. 68 g Rb 2 O 1 mole 186. 94 g 6. 02 x 1023 f. units Rb 2 O 1 mole = 4. 083 x 1022 f. units Rb 2 O
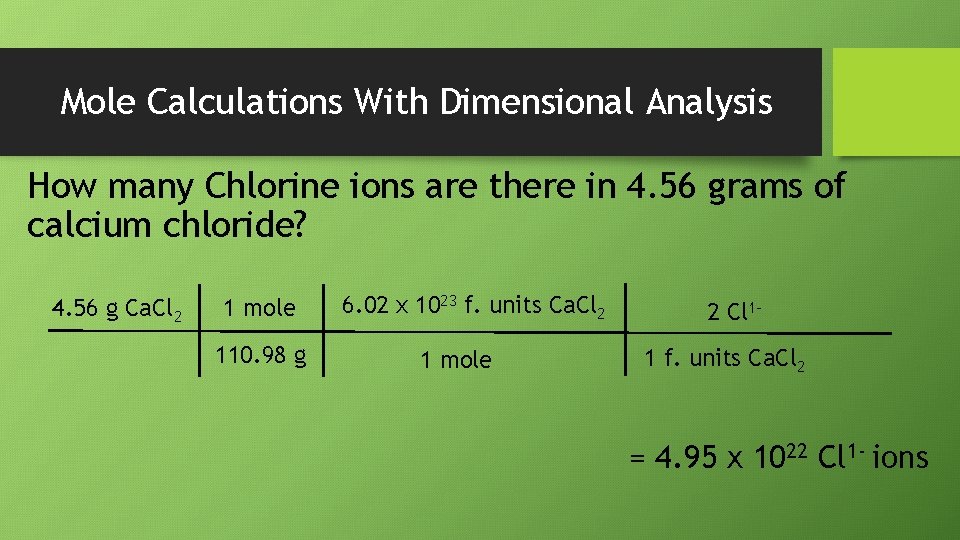
Mole Calculations With Dimensional Analysis How many Chlorine ions are there in 4. 56 grams of calcium chloride? 4. 56 g Ca. Cl 2 1 mole 110. 98 g 6. 02 x 1023 f. units Ca. Cl 2 1 mole 2 Cl 11 f. units Ca. Cl 2 = 4. 95 x 1022 Cl 1 - ions
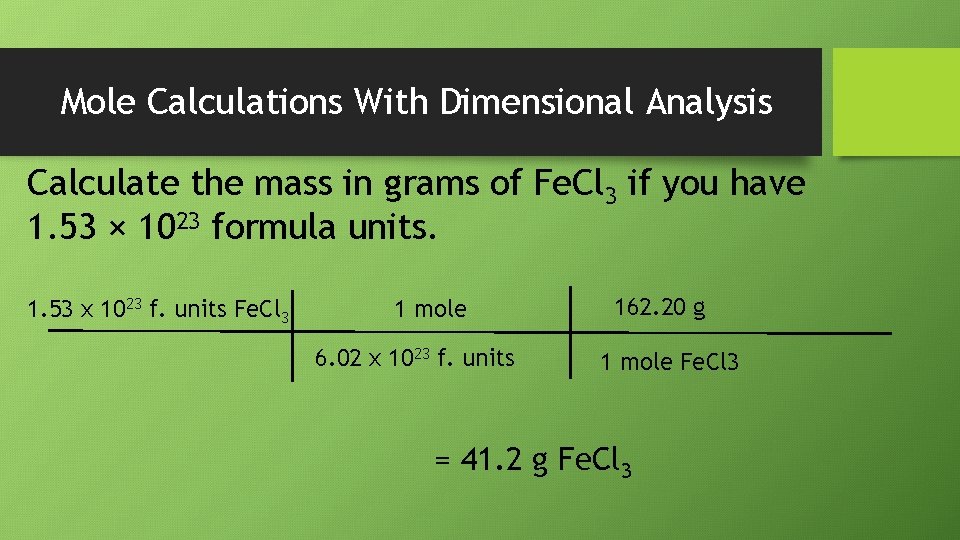
Mole Calculations With Dimensional Analysis Calculate the mass in grams of Fe. Cl 3 if you have 1. 53 × 1023 formula units. 1. 53 x 1023 f. units Fe. Cl 3 1 mole 6. 02 x 1023 f. units 162. 20 g 1 mole Fe. Cl 3 = 41. 2 g Fe. Cl 3

YOUR TURN TO TRY • How many formula units are there in 450 g of Na 2 SO 4? • How many grams are there in 7. 5 x 1023 formula units of H 2 SO 4? • How many formula units are there in 230 g of Co. Cl 2? • How many grams are there in 1. 3 x 1024 formula units of (NH 4)2 S? • How many lithium ions are there in 156. 7 g of lithium nitride? • What is the mass of 6. 8 x 1022 formula units of Sr. HSO 4? • How many ammonium ions are in 85. 2 g of ammonium phosphate?
- Slides: 11