THE MOLE INTRO VIDEO WORDS THAT REPRESENT NUMBERS

THE MOLE INTRO VIDEO

WORDS THAT REPRESENT NUMBERS • We all know a few words that represent numbers, like dozen and pair. • Dozen means that there are 12 of whatever the item is; doughnuts, eggs, pencils, etc. • Pair means that there are 2 of whatever the item is; socks, mittens, apples, etc. • These numbers are convenient for whatever they are counting, it is reasonable to have a dozen doughnuts and a pair of socks. • In chemistry, these numbers are way too small to count items as small as atoms so we use a larger grouping called the MOLE. • Mole, like dozen or pair, is just a word that represents a number, a very large number.

AVOGADRO’S NUMBER • The mole has a magnitude of 6. 022 x 1023 also referred to as Avogadro’s Number (NA) • How big is this number, watch the TEDEd video to get an idea. • You can also read the Green Pea analogy.

MOLE CALCULATIONS SIMPLIFIED • I’m going to take you through a few simple calculation to show you how easy these calculations are. • Let’s start with a simple dozen calculation. • If you go to the local Tim Horton’s drive through and order 3. 0 dozen doughnuts, how many doughnuts did you get?

36 DOUGHNUTS • The calculation is simple, I’m more interested in the problem solving process. • ? doughnuts = 3. 0 dozen X 12 doughnuts/dozen • = 36 doughnuts (the units of dozen cancel out) • The dozen is defined as 12 items/dozen

SAME CALCULATION USING MOLE • If a beaker contains 3. 0 mole of sugar, how many molecules of sugar are in the beaker? • Remember, mole is just a number like dozen, just way bigger.

1. 8 X 24 10 MOLECULES OF SUGAR • Where did that come from? • ? Molecules of sugar = 3. 0 mole x 6. 022 x 1023 molecules of sugar/mole • = 1. 8 x 1024 molecules of sugar (units of mole cancel out) • The mole is defined as 6. 022 x 1023 items/mole • Next slide will show you how to input the numbers into your calculator.
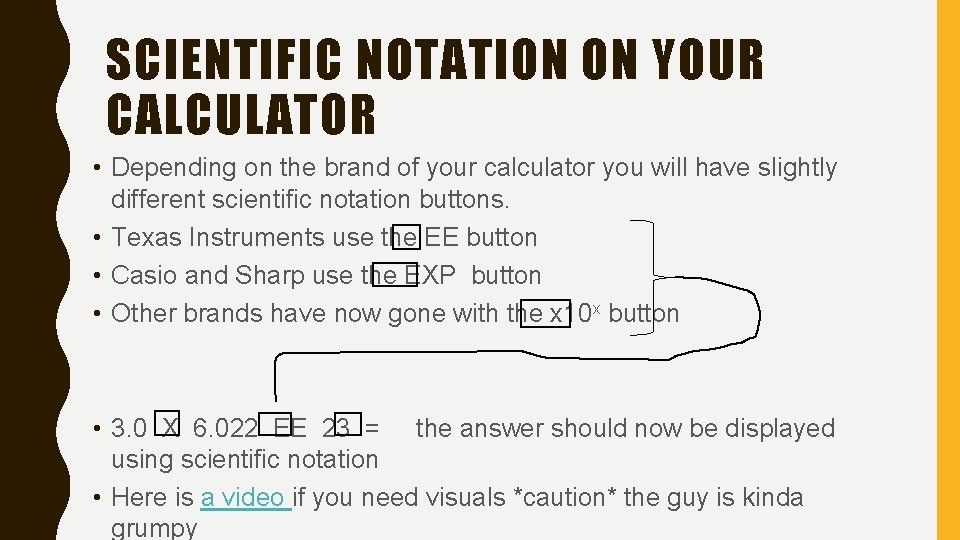
SCIENTIFIC NOTATION ON YOUR CALCULATOR • Depending on the brand of your calculator you will have slightly different scientific notation buttons. • Texas Instruments use the EE button • Casio and Sharp use the EXP button • Other brands have now gone with the x 10 x button • 3. 0 X 6. 022 EE 23 = the answer should now be displayed using scientific notation • Here is a video if you need visuals *caution* the guy is kinda grumpy

MOLE CALCULATION PRACTICE • How many particles are in the following samples: 1. 2. 5 moles of copper atoms 2. 4. 75 moles of water 3. 0. 0246 moles of Na. Cl • Answers are on the next slide with proper sig figs

ANSWERS 1. ? copper atoms = 2. 5 mole x 6. 022 x 1023 copper atoms/mole = 1. 5 x 1024 copper atoms (2 sig figs) 2. ? water molecules = 4. 75 mole x 6. 022 x 1023 water molecules/mole = 2. 86 x 1024 water molecules (3 sig figs) 3. ? Na. Cl formula units = 0. 0246 mole x 6. 022 x 1023 Na. Cl FU/mole = 1. 48 x 1022 Na. Cl formula units (3 sig figs) FU (Formula Units) is the unit for an ionic compound, like molecule for covalent compounds

GOING THE OTHER WAY • If you have 78 marbles in a bag, how many dozen marbles do you have?

6. 5 DOZEN MARBLES • ? Dozen = 78 marbles ÷ 12 marbles/dozen = 6. 5 dozen When dividing the units, think of dividing fractions since the unit of dozen is actually a fraction. Multiply by the reciprocal marbles ÷ marbles dozen = marbles x dozen marbles

BACK TO MOLES • A balloon is filled with 7. 352 x 1024 atoms of helium, how many moles of helium are in the balloon?

12. 21 MOLES OF HELIUM • ? moles = 7. 352 x 1024 atoms He ÷ 6. 022 x 1023 atoms He/mole = 12. 2085686 moles (rounds to 12. 21 moles 4 sig figs) atoms He ÷ atoms He mole = atoms He x mole atoms He
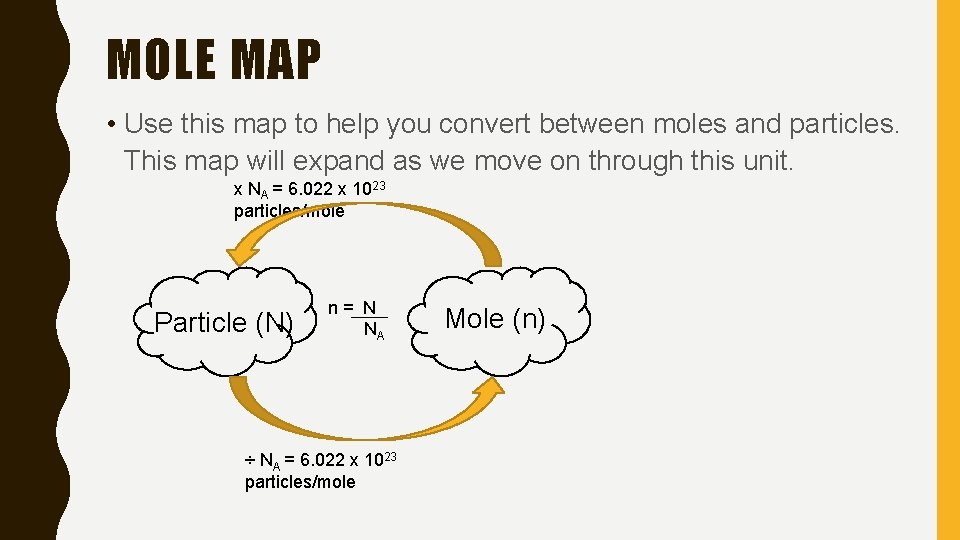
MOLE MAP • Use this map to help you convert between moles and particles. This map will expand as we move on through this unit. x NA = 6. 022 x 1023 particles/mole Particle (N) n= N NA ÷ NA = 6. 022 x 1023 particles/mole Mole (n)

PRACTICE • Here is a review video that may help with these calculations. There is a typo on the video at about 1: 40 she refers to molecules of CO 2 as atoms…oops • Tyler Dewitt also explains the calculations • 1) 2. 80 x 1024 atoms Si Moles • 2) 2. 17 x 1023 molecules Br 2 Moles • 3) 5. 14 x 1023 FU Mg. O Moles • 4) 3. 00 mol Sn Atoms • 5) 0. 400 mol KCl Formula units • 6) 7. 50 mol SO 2 Molecules • Check Your Understanding Quiz
- Slides: 16