The Mole Chapter 10 What is a Mole





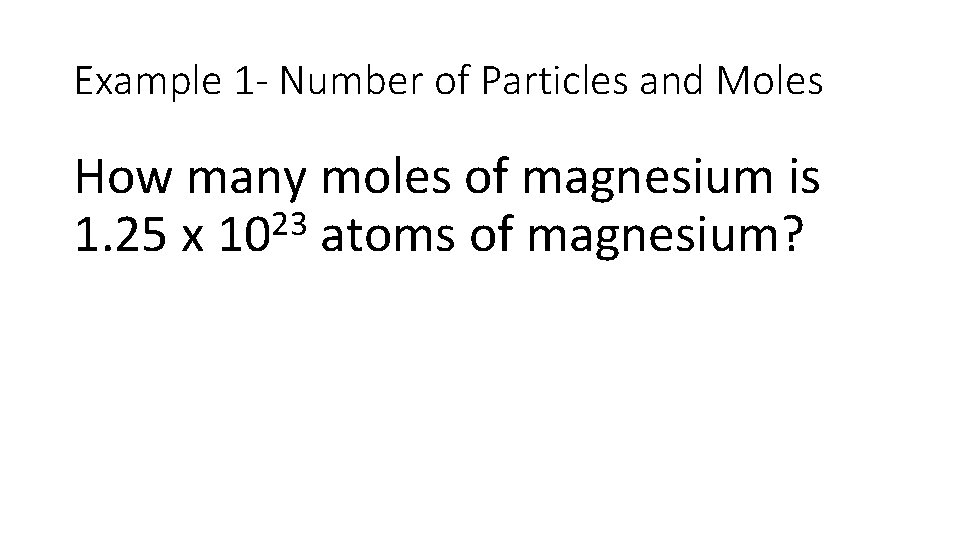




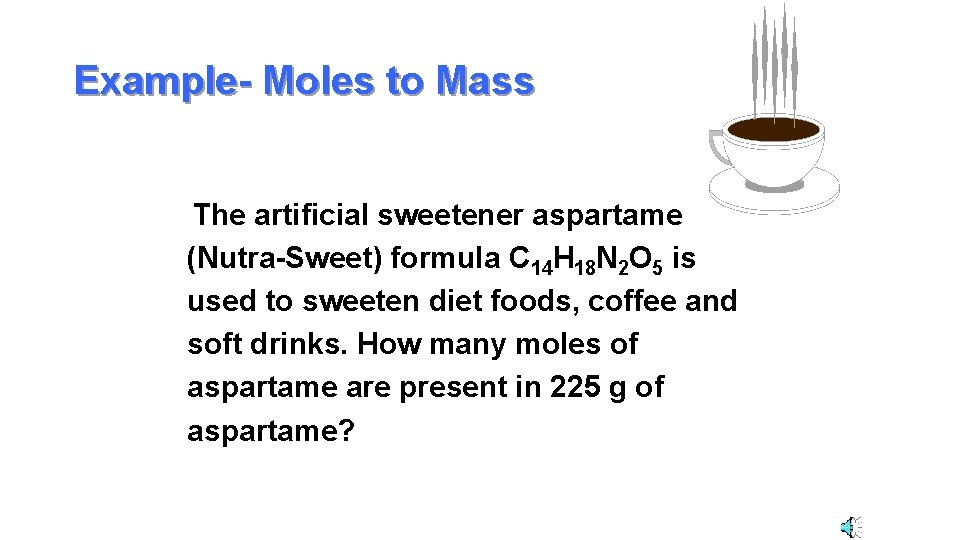







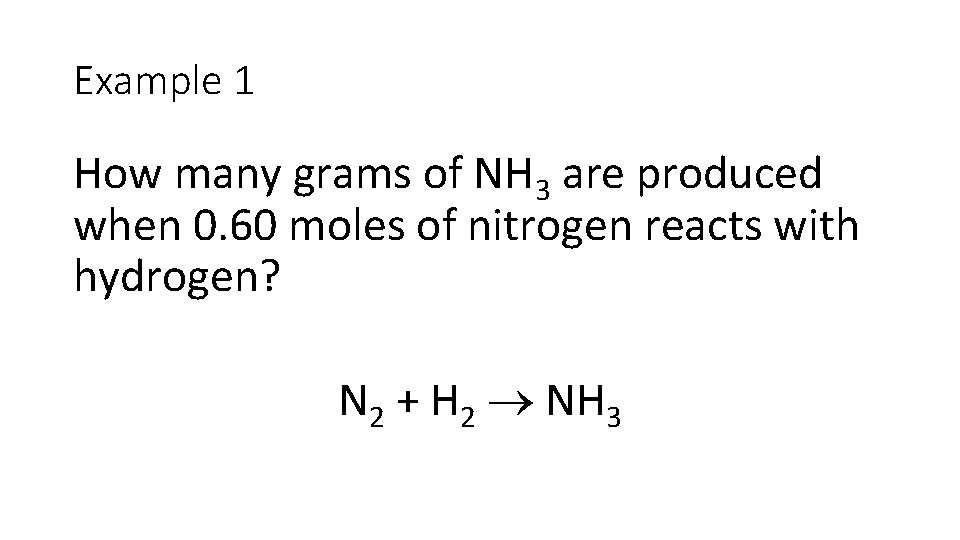
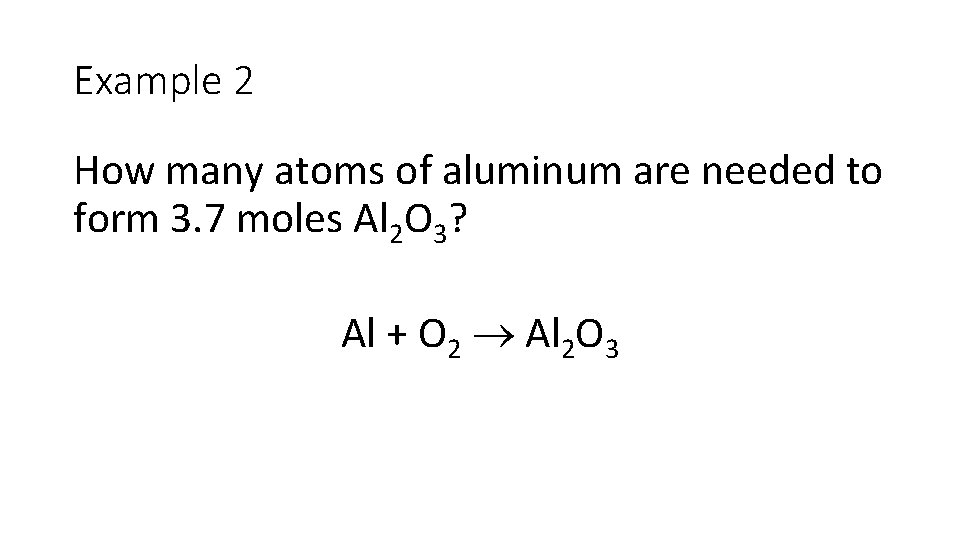


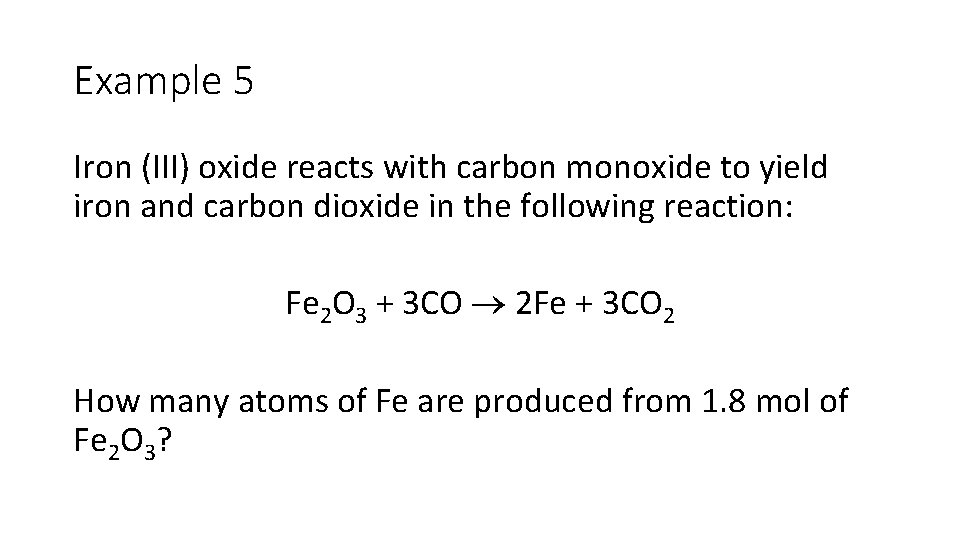


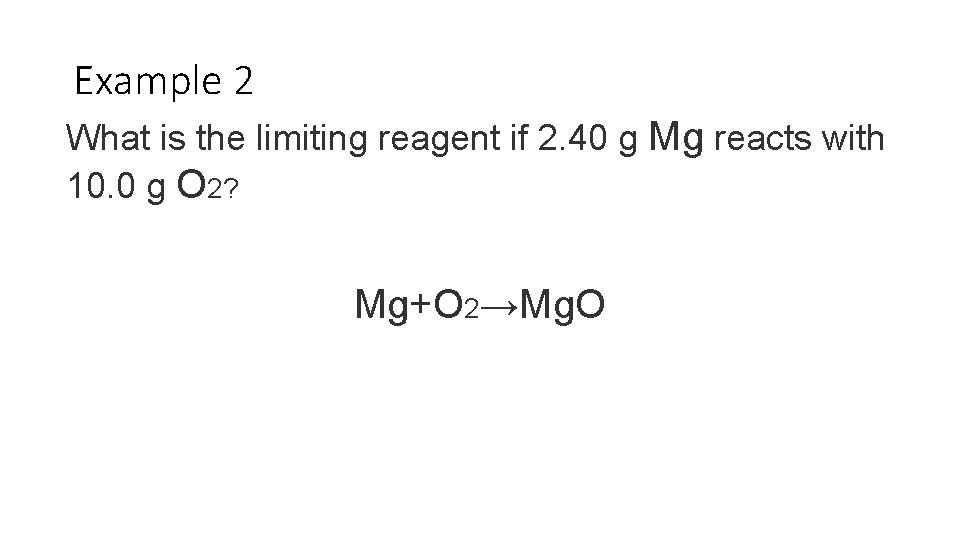
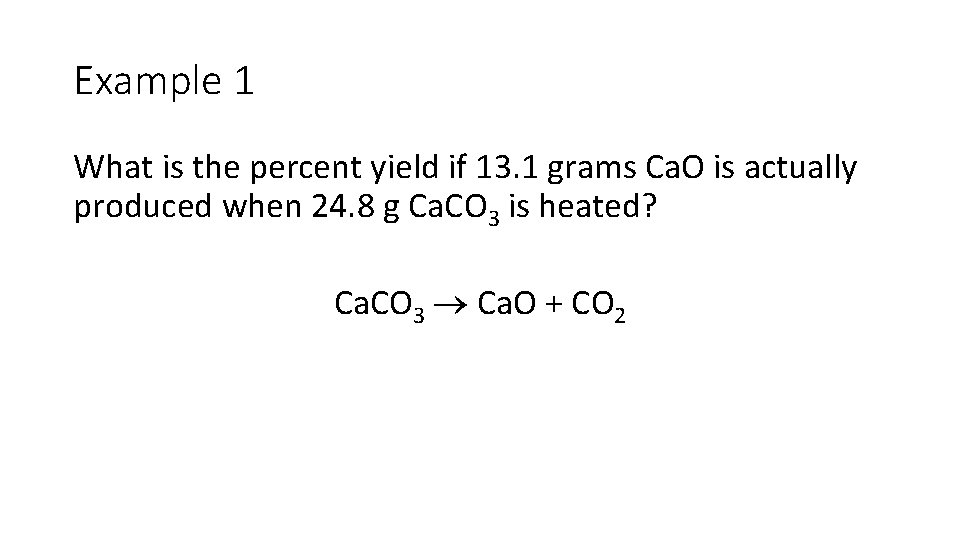
- Slides: 44

The Mole Chapter 10

What is a Mole? • A unit that is a specified number of particles (atom, molecule, ion, or formula unit) • Avogadro’s Number: 6. 02 x 1023 representative particles.

Just How Big is a Mole? • Enough soft drink cans to cover the surface of the earth to a depth of over 200 miles. • If you had Avogadro's number of unpopped popcorn kernels, and spread them across the United States of America, the country would be covered in popcorn to a depth of over 9 miles. • If we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count the atoms in one mole.

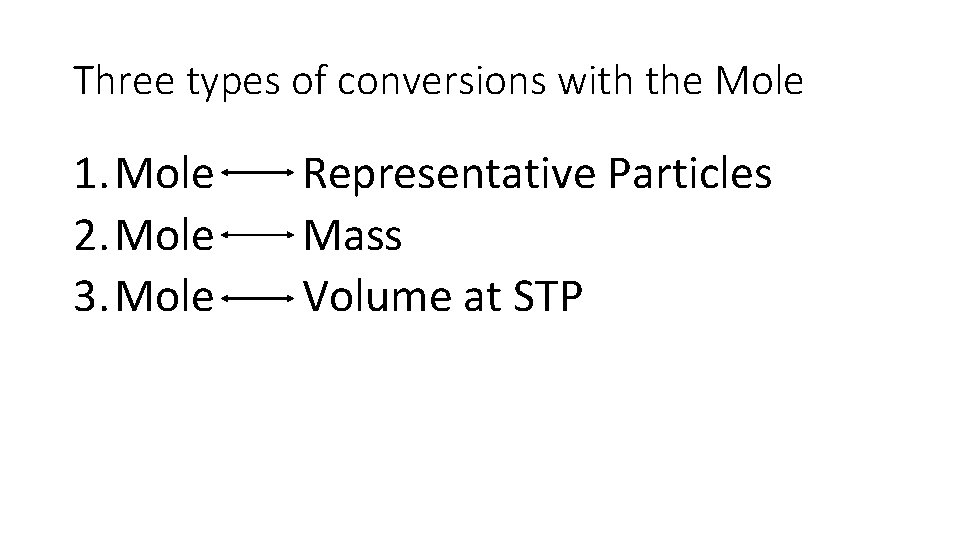
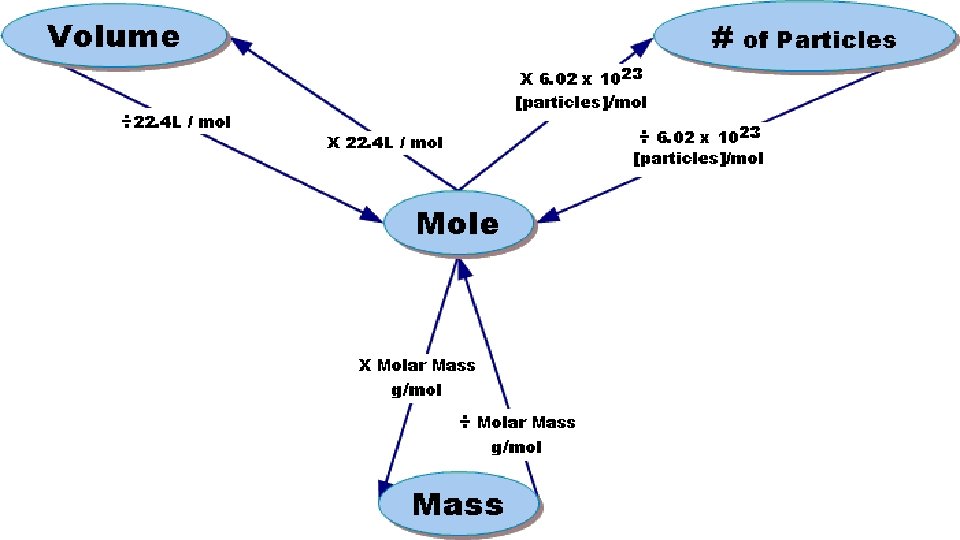
Three types of conversions with the Mole 1. Mole Representative Particles 2. Mole Mass 3. Mole Volume at STP

This is your task and you have to do it… You must find in your textbook the three conversion factors we are going to be using to convert back and forth.



The Mole • 1 dozen cookies = 12 cookies • 1 mole of cookies = 6. 02 X 1023 cookies • 1 dozen cars = 12 cars • 1 mole of cars = 6. 02 X 1023 cars • 1 dozen Al atoms = 12 Al atoms • 1 mole of Al atoms = 6. 02 X 1023 atoms Note that the NUMBER is always the same, but the MASS is very different!

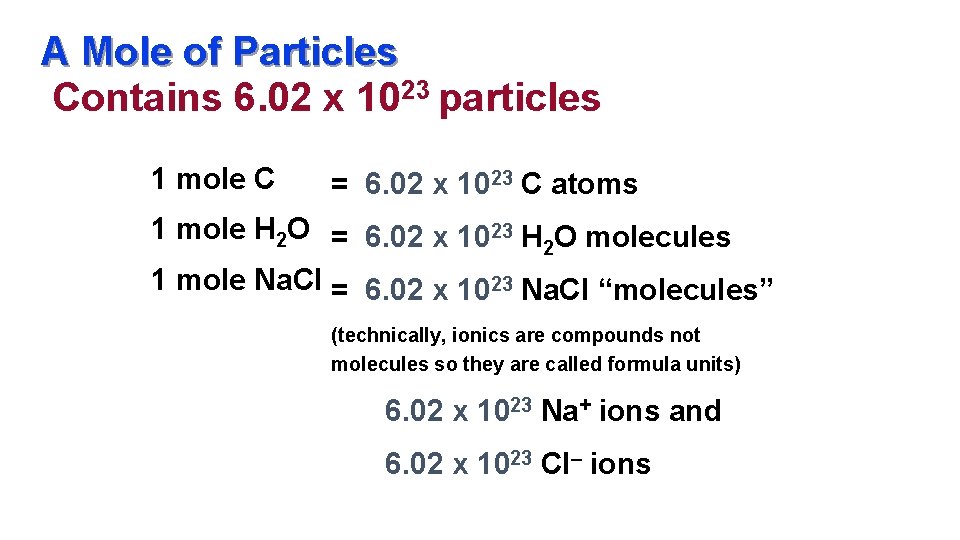
A Mole of Particles Contains 6. 02 x 1023 particles 1 mole C = 6. 02 x 1023 C atoms 1 mole H 2 O = 6. 02 x 1023 H O molecules 2 1 mole Na. Cl = 6. 02 x 1023 Na. Cl “molecules” (technically, ionics are compounds not molecules so they are called formula units) 6. 02 x 1023 Na+ ions and 6. 02 x 1023 Cl– ions

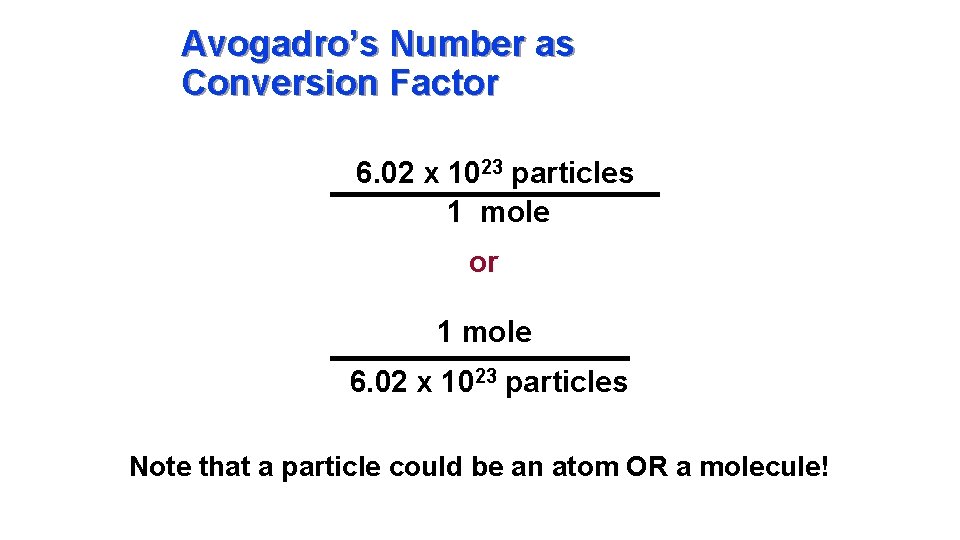
Avogadro’s Number as Conversion Factor 6. 02 x 1023 particles 1 mole or 1 mole 6. 02 x 1023 particles Note that a particle could be an atom OR a molecule!
Example 1 - Number of Particles and Moles How many moles of magnesium is 23 1. 25 x 10 atoms of magnesium?

Example 2 - Number of Particles and Moles 23 How many moles is 2. 80 x 10 atoms of silicon?

Converting Moles to Molar Mass • To convert from moles to mass or mass to moles you must find molar mass (mass of a mole) • Units used for molar mass: atomic mass units (amu) • The molar mass of an element is found on the periodic table • The molar mass of a compound you must solve (hahaha and if your writing this, sucka’)

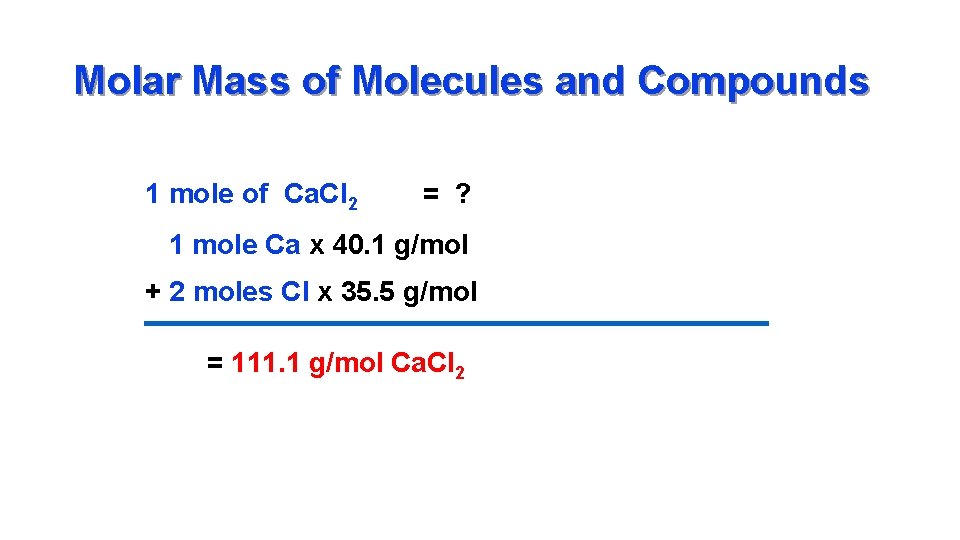
Molar Mass of Molecules and Compounds 1 mole of Ca. Cl 2 = ? 1 mole Ca x 40. 1 g/mol + 2 moles Cl x 35. 5 g/mol = 111. 1 g/mol Ca. Cl 2

Example- Molar Mass Prozac, C 17 H 18 F 3 NO, is a widely used antidepressant that inhibits the uptake of serotonin by the brain. Find its molar mass.

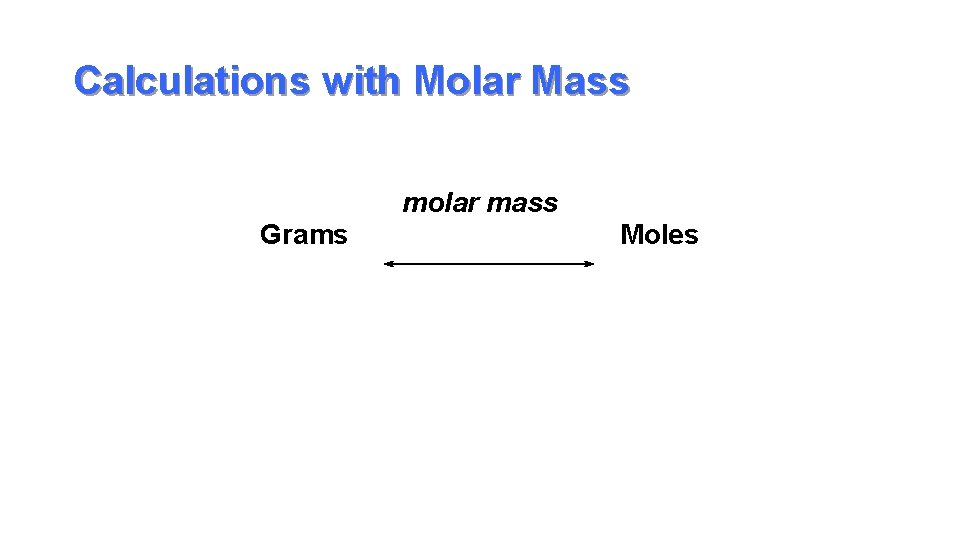
Calculations with Molar Mass molar mass Grams Moles

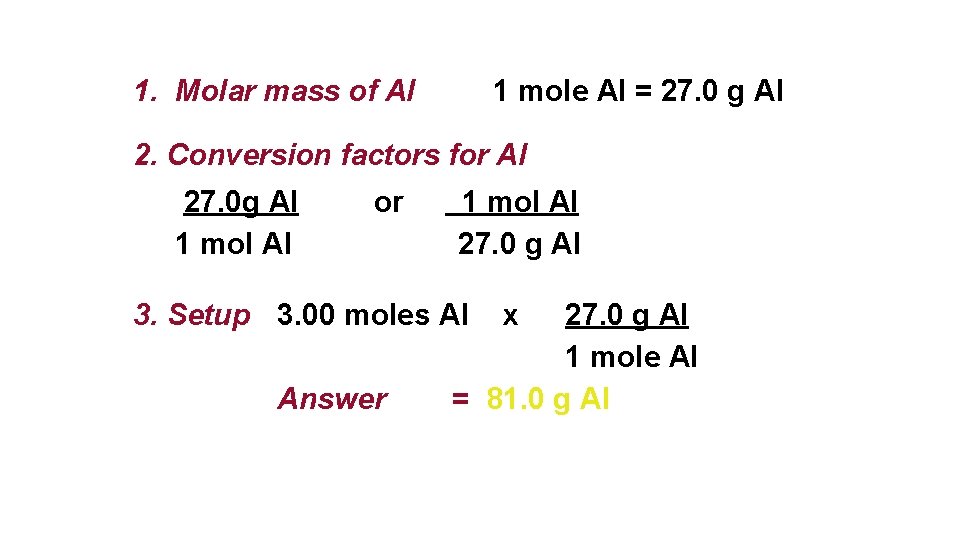
Converting Moles and Grams Aluminum is often used for the structure of light-weight bicycle frames. How many grams of Al are in 3. 00 moles of Al? 3. 00 moles Al ? g Al

1. Molar mass of Al 1 mole Al = 27. 0 g Al 2. Conversion factors for Al 27. 0 g Al or 1 mol Al 27. 0 g Al 3. Setup 3. 00 moles Al x 27. 0 g Al 1 mole Al Answer = 81. 0 g Al
Example- Moles to Mass The artificial sweetener aspartame (Nutra-Sweet) formula C 14 H 18 N 2 O 5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 g of aspartame?

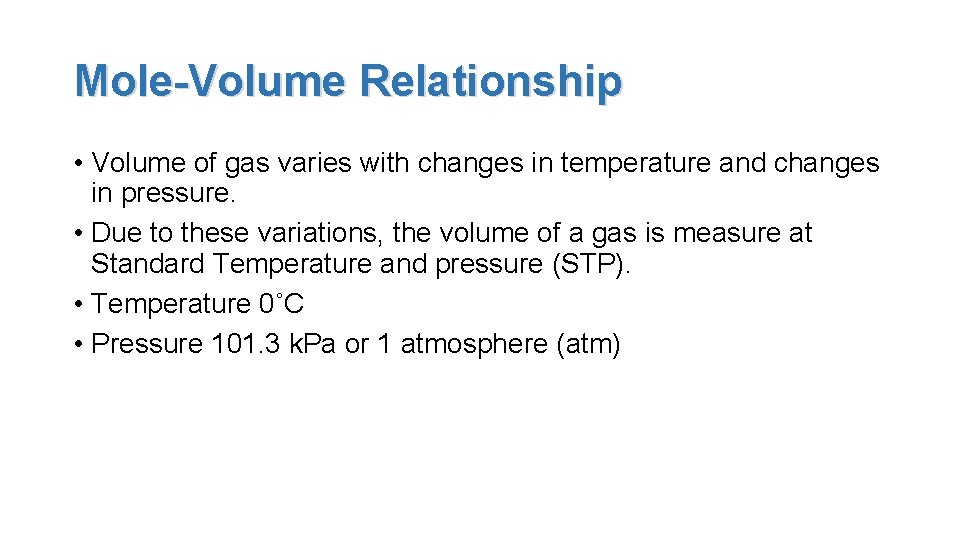
Mole-Volume Relationship • Volume of gas varies with changes in temperature and changes in pressure. • Due to these variations, the volume of a gas is measure at Standard Temperature and pressure (STP). • Temperature 0˚C • Pressure 101. 3 k. Pa or 1 atmosphere (atm)

Conversion Factor

Example Sulfur dioxide is a gas produced by burning coal. Determine the volume, in liters of 0. 60 mole SO 2 gas at STP.

Example At STP, what volume is occupied by each of the following gases? 1. 6. 7 mol H 2 2. 2. 56 mol CH 4

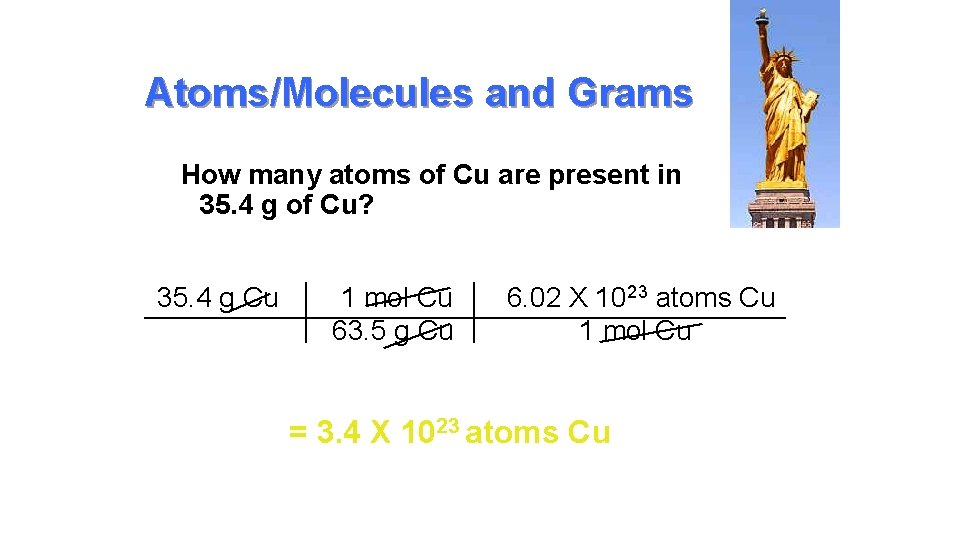
Atoms/Molecules and Grams • Since 6. 02 X 1023 particles = 1 mole AND 1 mole = molar mass (grams) • You can convert atoms/molecules to moles and then moles to grams! (Two step process) • You can’t go directly from atoms to grams!!!! You MUST go thru MOLES. • That’s like asking 2 dozen cookies weigh how many ounces if 1 cookie weighs 4 oz? You have to convert to dozen first!

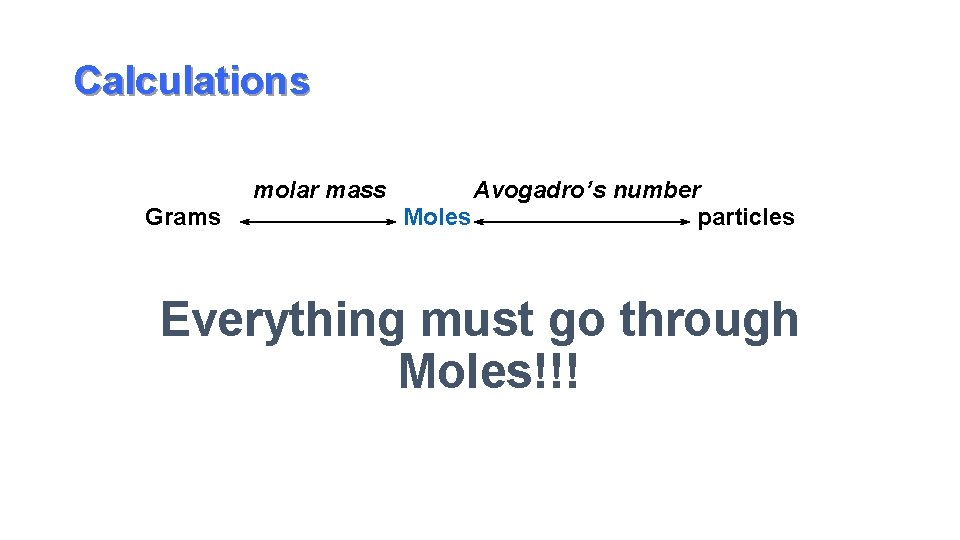
Calculations Grams molar mass Avogadro’s number Moles particles Everything must go through Moles!!!

Atoms/Molecules and Grams How many atoms of Cu are present in 35. 4 g of Cu? 35. 4 g Cu 1 mol Cu 6. 02 X 1023 atoms Cu 63. 5 g Cu 1 mol Cu = 3. 4 X 1023 atoms Cu

Learning Check! How many atoms of K are present in 78. 4 g of K?

Learning Check! What is the mass (in grams) of 1. 20 X 1024 molecules of glucose (C 6 H 12 O 6)?

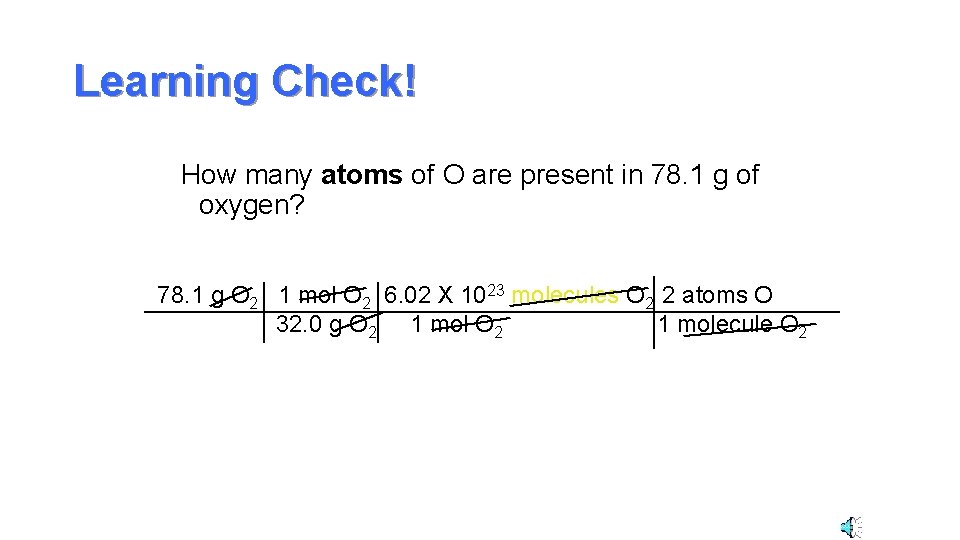
Learning Check! How many atoms of O are present in 78. 1 g of oxygen? 78. 1 g O 2 1 mol O 2 6. 02 X 1023 molecules O 2 2 atoms O 32. 0 g O 2 1 mol O 2 1 molecule O 2

Stoichiometry!!! • The calculation of quantities in chemical reactions • We will use balanced chemical equations to calculate how much reactant is needed or how much product is produced in a reaction.

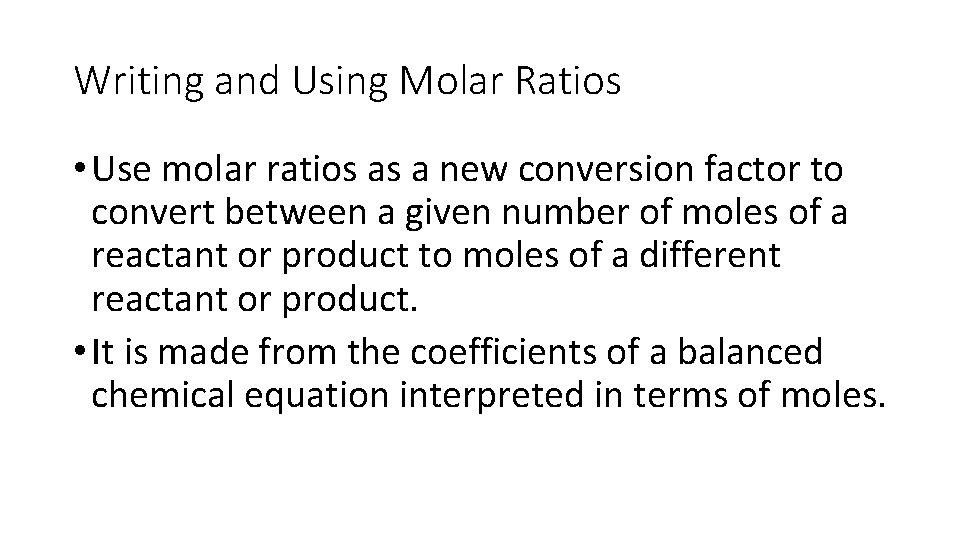
Writing and Using Molar Ratios • Use molar ratios as a new conversion factor to convert between a given number of moles of a reactant or product to moles of a different reactant or product. • It is made from the coefficients of a balanced chemical equation interpreted in terms of moles.
Example 1 How many grams of NH 3 are produced when 0. 60 moles of nitrogen reacts with hydrogen? N 2 + H 2 NH 3
Example 2 How many atoms of aluminum are needed to form 3. 7 moles Al 2 O 3? Al + O 2 Al 2 O 3

Example 3 What volume (L) of oxygen are required to react completely with 14. 8 moles Al? 4 Al + 3 O 2 2 Al 2 O 3

Example 4 How many grams of Al 2 O 3 are formed when 0. 78 mol O 2 reacts with aluminum? 4 Al + 3 O 2 2 Al 2 O 3
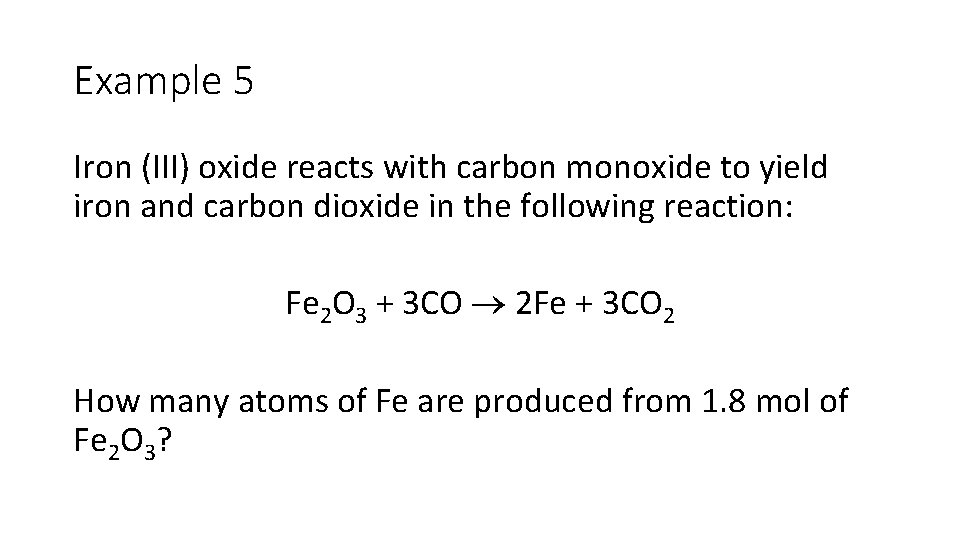
Example 5 Iron (III) oxide reacts with carbon monoxide to yield iron and carbon dioxide in the following reaction: Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 How many atoms of Fe are produced from 1. 8 mol of Fe 2 O 3?

Limiting/ Excess Reagent • In a chemical reaction, the reactant that determines the amount of product that can be formed by a reaction is the limiting reagent. • The reactant that is not completely used up in a reaction is called the excess reagent.

How to find limiting Reagent Approach 2: Find the limiting reagent by calculating and comparing the amount of product each reactant will produce. 1. Balance the chemical equation 2. Convert the given information into moles (if necessary). 3. Use stoichiometry for each individual reactant to find the mass of product produced. 4. The reactant that produces a lesser amount of product is the limiting reagent. 5. The reactant that produces a larger amount of product is the excess reagent. 6. To find the amount of remaining excess reactant, subtract the mass of excess reagent consumed from the total mass of excess reagent given.

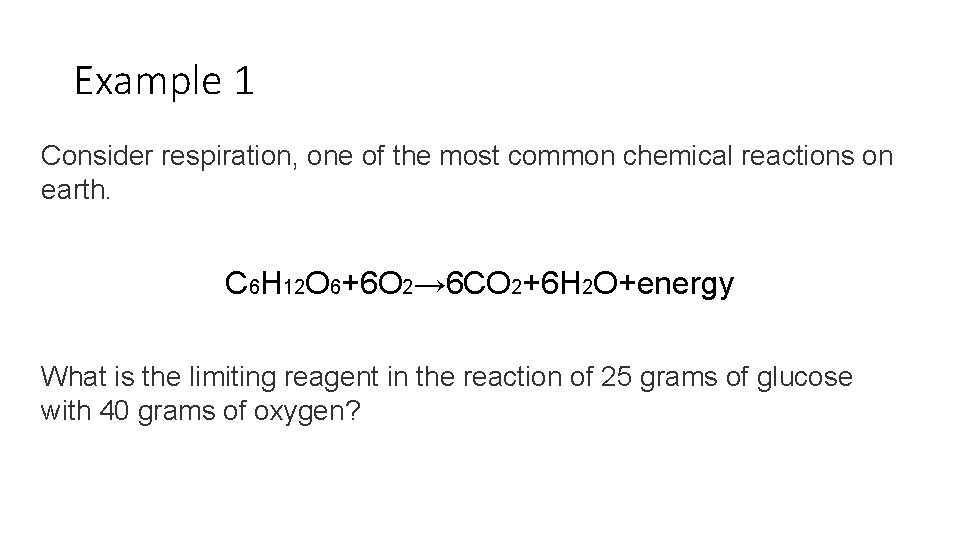
Example 1 Consider respiration, one of the most common chemical reactions on earth. C 6 H 12 O 6+6 O 2→ 6 CO 2+6 H 2 O+energy What is the limiting reagent in the reaction of 25 grams of glucose with 40 grams of oxygen?
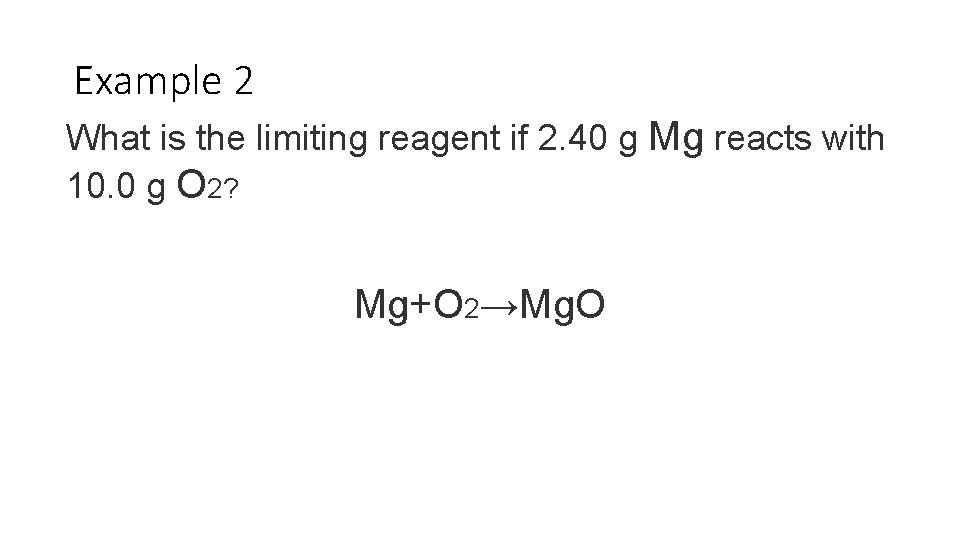
Example 2 What is the limiting reagent if 2. 40 g Mg reacts with 10. 0 g O 2? Mg+O 2→Mg. O

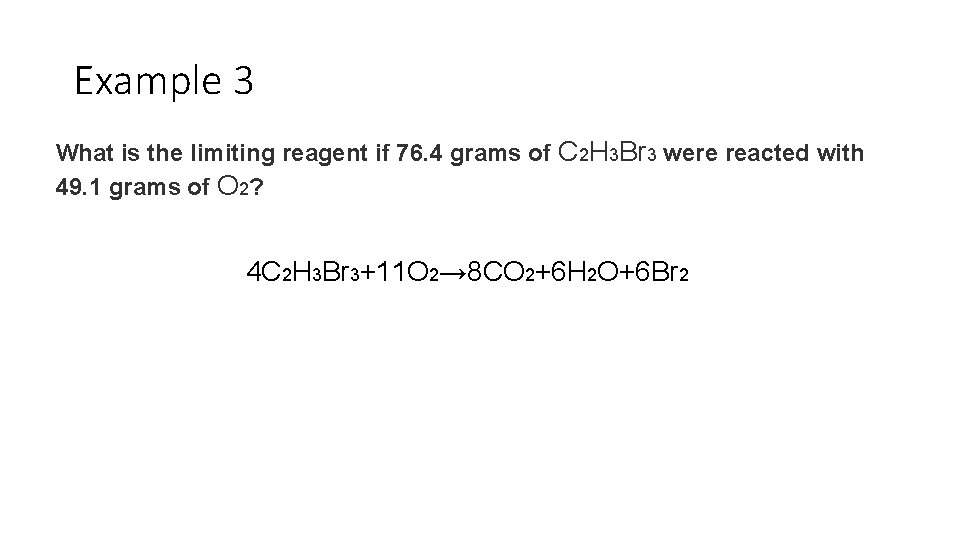
Example 3 What is the limiting reagent if 76. 4 grams of C 2 H 3 Br 3 were reacted with 49. 1 grams of O 2? 4 C 2 H 3 Br 3+11 O 2→ 8 CO 2+6 H 2 O+6 Br 2

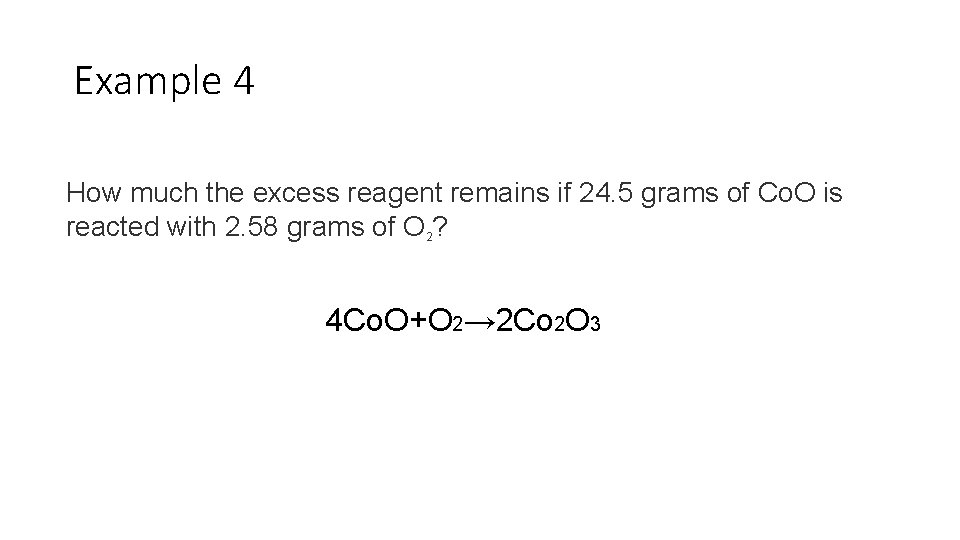
Example 4 How much the excess reagent remains if 24. 5 grams of Co. O is reacted with 2. 58 grams of O 2? 4 Co. O+O 2→ 2 Co 2 O 3

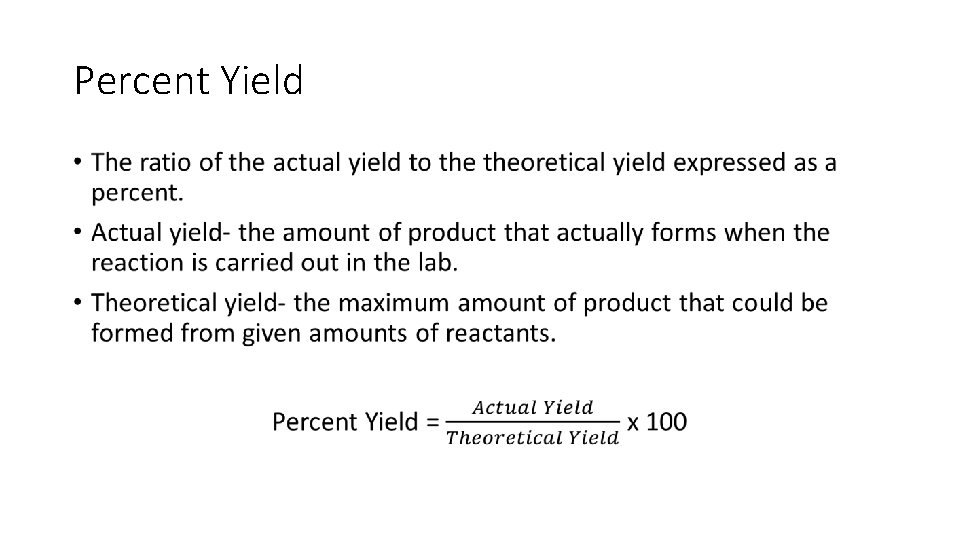
Percent Yield •
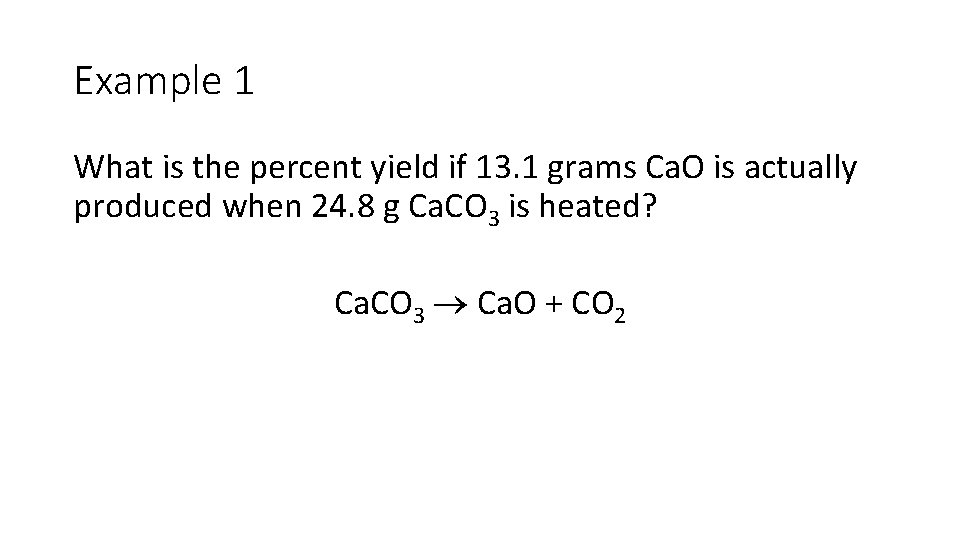
Example 1 What is the percent yield if 13. 1 grams Ca. O is actually produced when 24. 8 g Ca. CO 3 is heated? Ca. CO 3 Ca. O + CO 2