The Mole and Stoichiometry Part 1 Chapter 9




- Slides: 13

The Mole and Stoichiometry Part 1 Chapter 9

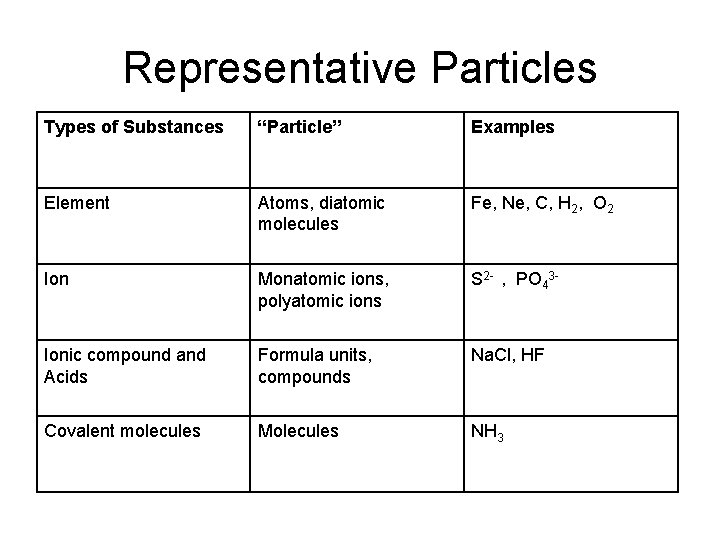
Representative Particles Types of Substances “Particle” Examples Element Atoms, diatomic molecules Fe, Ne, C, H 2, O 2 Ion Monatomic ions, polyatomic ions S 2 - , PO 43 - Ionic compound and Acids Formula units, compounds Na. Cl, HF Covalent molecules Molecules NH 3

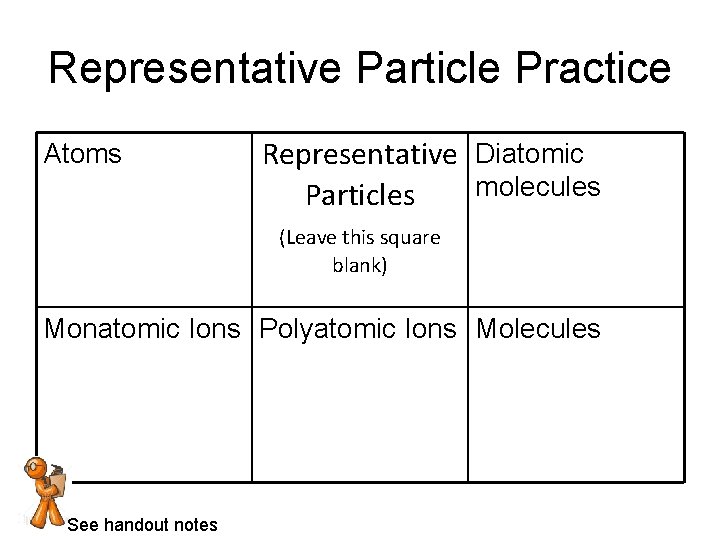
Representative Particle Practice Atoms Representative Diatomic molecules Particles (Leave this square blank) Monatomic Ions Polyatomic Ions Molecules See handout notes

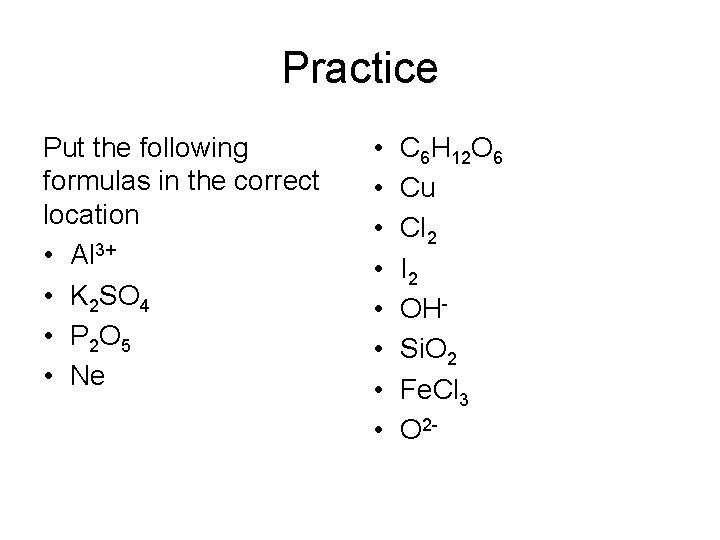
Practice Put the following formulas in the correct location • Al 3+ • K 2 SO 4 • P 2 O 5 • Ne • • C 6 H 12 O 6 Cu Cl 2 I 2 OHSi. O 2 Fe. Cl 3 O 2 -

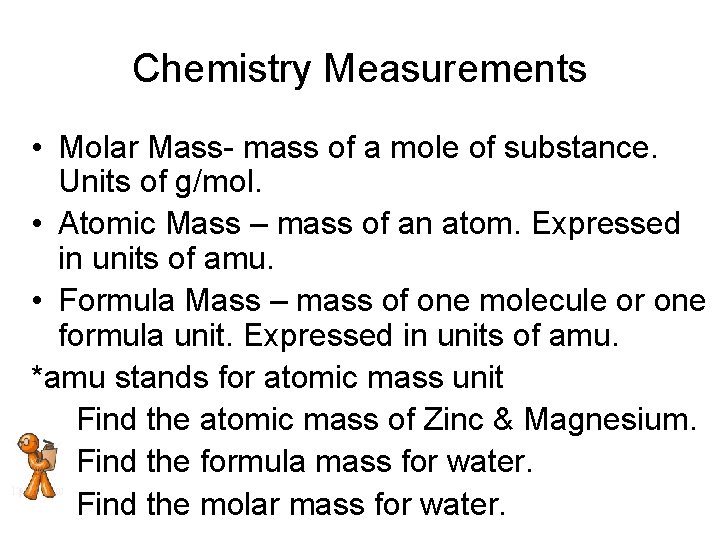
Chemistry Measurements • Molar Mass- mass of a mole of substance. Units of g/mol. • Atomic Mass – mass of an atom. Expressed in units of amu. • Formula Mass – mass of one molecule or one formula unit. Expressed in units of amu. *amu stands for atomic mass unit Find the atomic mass of Zinc & Magnesium. Find the formula mass for water. Find the molar mass for water.

Subatomic to Macro • What is the desired unit in a lab for mass? » GRAM • Therefore, we need to relate the atomic mass (amu) of an element to an amount of the element in grams • The MOLE relates these two!

The Mole • Think of the mole as a bridge…. . • It connects the subatomic world of atoms and molecules which are invisible to the macroscopic world of elements and compounds that are visible and who’s mass can be determined.

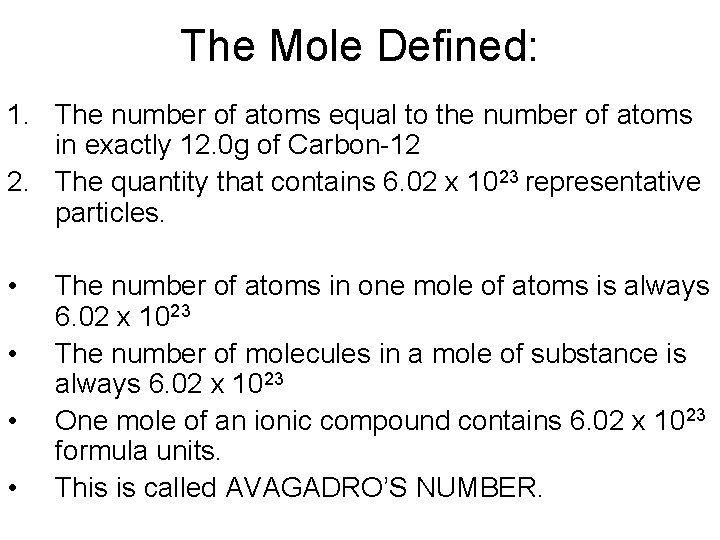
The Mole Defined: 1. The number of atoms equal to the number of atoms in exactly 12. 0 g of Carbon-12 2. The quantity that contains 6. 02 x 1023 representative particles. • • The number of atoms in one mole of atoms is always 6. 02 x 1023 The number of molecules in a mole of substance is always 6. 02 x 1023 One mole of an ionic compound contains 6. 02 x 1023 formula units. This is called AVAGADRO’S NUMBER.

Amedeo Avagadro • Avogadro proposed his hypothesis in 1811. At that time there was no data at all on the number of particles in a mole, or an agreement on any atomic weights or the standard. The first measurements which could give an approximate value for Avogadro's number were observations of Brownian motion by Robert Brown in 1827.

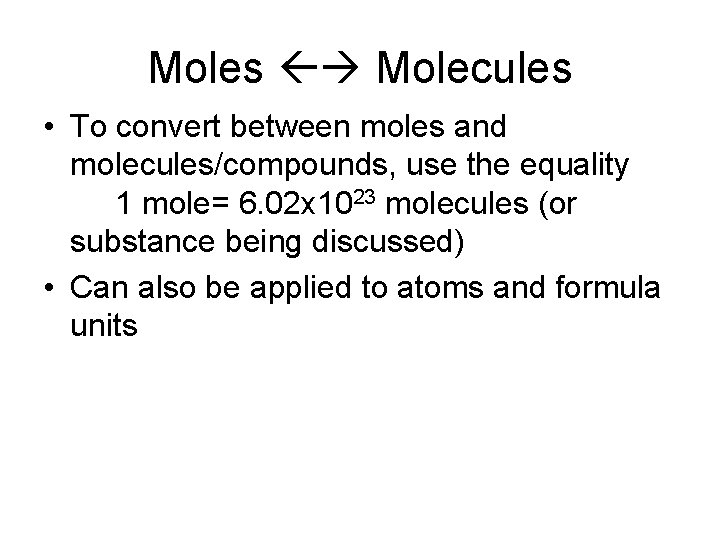
Moles Molecules • To convert between moles and molecules/compounds, use the equality 1 mole= 6. 02 x 1023 molecules (or substance being discussed) • Can also be applied to atoms and formula units

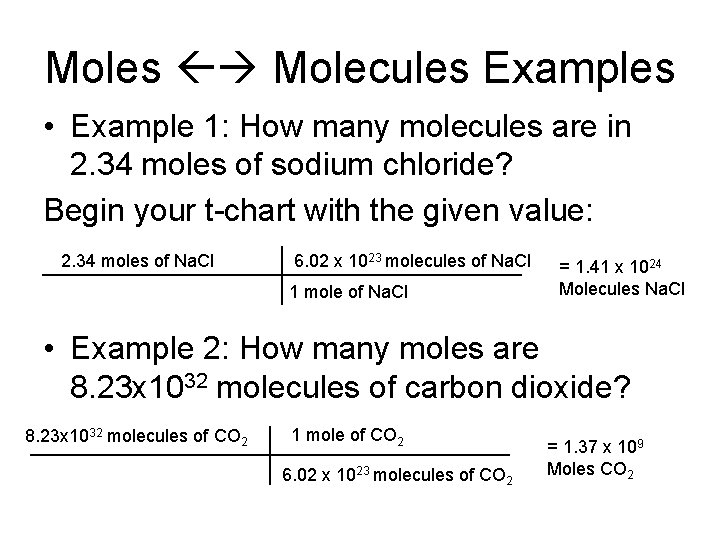
Moles Molecules Examples • Example 1: How many molecules are in 2. 34 moles of sodium chloride? Begin your t-chart with the given value: 2. 34 moles of Na. Cl 6. 02 x 1023 molecules of Na. Cl 1 mole of Na. Cl = 1. 41 x 1024 Molecules Na. Cl • Example 2: How many moles are 8. 23 x 1032 molecules of carbon dioxide? 8. 23 x 1032 molecules of CO 2 1 mole of CO 2 6. 02 x 1023 molecules of CO 2 = 1. 37 x 109 Moles CO 2

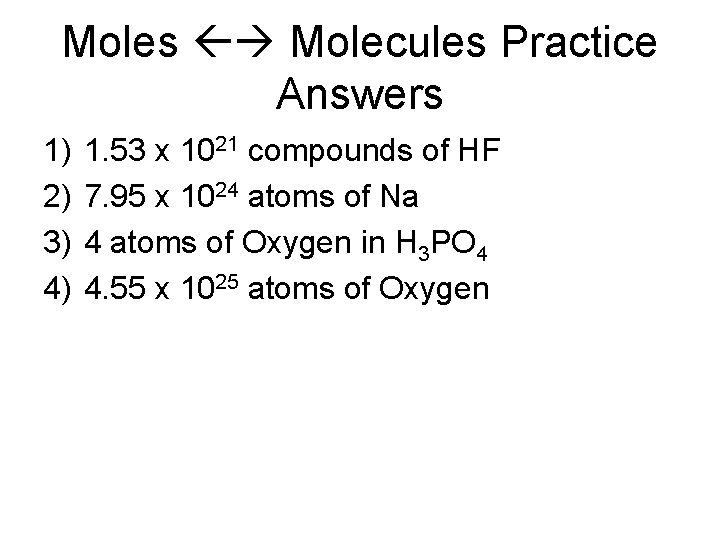
Moles Molecules Practice 1. How many moles are in 9. 21 x 1044 compounds of Hydrofluoric acid? 2. How many atoms of sodium are in 13. 2 moles? 3. How many atoms of oxygen are in Phosphoric acid? 4. How many atoms of oxygen are in 18. 9 moles of phosphoric acid (hint convert to moles first then convert to atoms)?

Moles Molecules Practice Answers 1) 2) 3) 4) 1. 53 x 1021 compounds of HF 7. 95 x 1024 atoms of Na 4 atoms of Oxygen in H 3 PO 4 4. 55 x 1025 atoms of Oxygen