The Mole and Avogadros Worksheet 1 1 1



- Slides: 6

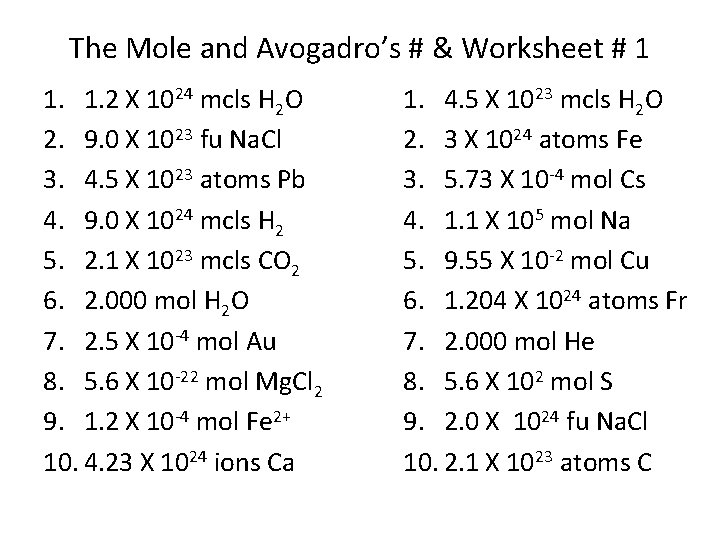
The Mole and Avogadro’s # & Worksheet # 1 1. 1. 2 X 1024 mcls H 2 O 2. 9. 0 X 1023 fu Na. Cl 3. 4. 5 X 1023 atoms Pb 4. 9. 0 X 1024 mcls H 2 5. 2. 1 X 1023 mcls CO 2 6. 2. 000 mol H 2 O 7. 2. 5 X 10 -4 mol Au 8. 5. 6 X 10 -22 mol Mg. Cl 2 9. 1. 2 X 10 -4 mol Fe 2+ 10. 4. 23 X 1024 ions Ca 1. 4. 5 X 1023 mcls H 2 O 2. 3 X 1024 atoms Fe 3. 5. 73 X 10 -4 mol Cs 4. 1. 1 X 105 mol Na 5. 9. 55 X 10 -2 mol Cu 6. 1. 204 X 1024 atoms Fr 7. 2. 000 mol He 8. 5. 6 X 102 mol S 9. 2. 0 X 1024 fu Na. Cl 10. 2. 1 X 1023 atoms C

Gram Formula Mass Answers 1. potassium permanganate 2. potassium chloride 3. sodium sulfate 4. calcium nitrate 5. aluminum sulfate 6. ammonium phosphate 7. mmagnesium phosphate 8. carbonic acid 9. zinc acetate 10. lead 1. 158. 04 g/mol 2. 74. 55 g/mol 3. 142. 05 g/mol 4. 164. 10 g/mol 5. 342. 17 g/mol 6. 149. 12 g/mol 7. 262. 87 g/mol 8. 62. 03 g/mol 9. 183. 48 g/mol 10. 207. 20 g/mol

11. barium chlorate 12. iron (III) sulfite 13. strontium 14. bromine 15. copper (II) sulfate pentahydrate 11. 62. 03 g/mol 12. 351. 91 g/mol 13. 87. 62 g/mol 14. 159. 80 g/mol 15. 249. 70 g/mol

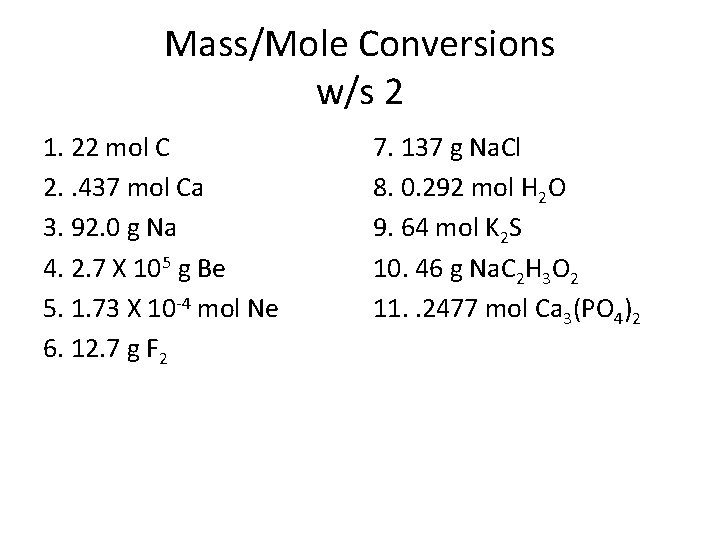
Mass/Mole Conversions w/s 2 1. 22 mol C 2. . 437 mol Ca 3. 92. 0 g Na 4. 2. 7 X 105 g Be 5. 1. 73 X 10 -4 mol Ne 6. 12. 7 g F 2 7. 137 g Na. Cl 8. 0. 292 mol H 2 O 9. 64 mol K 2 S 10. 46 g Na. C 2 H 3 O 2 11. . 2477 mol Ca 3(PO 4)2

Worksheet # 3 Mass Particles 1. 6 X 1021 atoms Ag 2. 2. 5 X 10 -16 g Na 3. 2. 43 g H 2 O 4. 2. 3 X 1023 atoms Li 5. 2. 414 X 1024 mcls CH 3 OH 6. 2 X 10 -23 g B

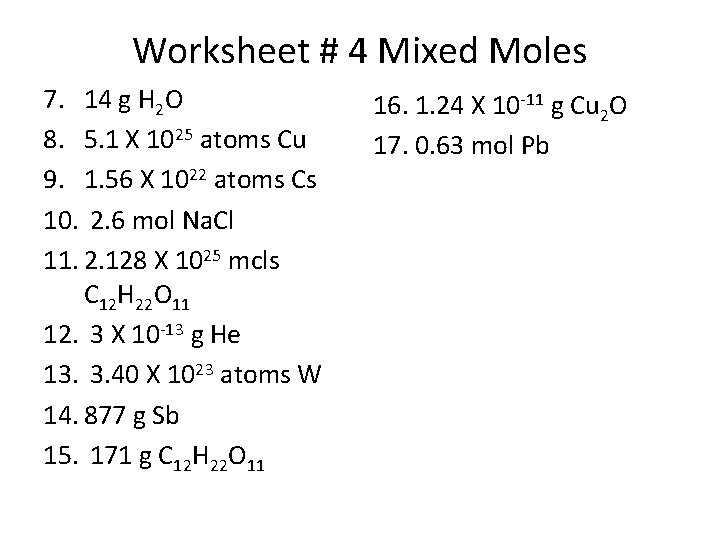
Worksheet # 4 Mixed Moles 7. 14 g H 2 O 8. 5. 1 X 1025 atoms Cu 9. 1. 56 X 1022 atoms Cs 10. 2. 6 mol Na. Cl 11. 2. 128 X 1025 mcls C 12 H 22 O 11 12. 3 X 10 -13 g He 13. 3. 40 X 1023 atoms W 14. 877 g Sb 15. 171 g C 12 H 22 O 11 16. 1. 24 X 10 -11 g Cu 2 O 17. 0. 63 mol Pb