The Mole 1 dozen 12 1 gross 144




- Slides: 7


The Mole 1 dozen = 12 1 gross = 144 1 ream = 500 1 mole = 6. 022 x 1023 There are exactly 12 grams of carbon-12 in one mole of carbon-12.

Avogadro’s Number 6. 022 x 1023 is called “Avogadro’s Number” in honor of the Italian chemist Amadeo Avogadro (1776 -1855). I didn’t discover it. Its just named after me! Amadeo Avogadro

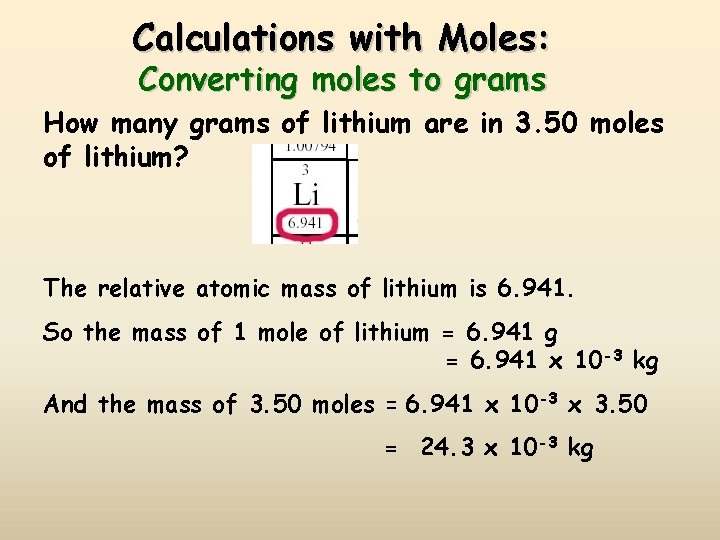
Calculations with Moles: Converting moles to grams How many grams of lithium are in 3. 50 moles of lithium? The relative atomic mass of lithium is 6. 941. So the mass of 1 mole of lithium = 6. 941 g = 6. 941 x 10 -3 kg And the mass of 3. 50 moles = 6. 941 x 10 -3 x 3. 50 = 24. 3 x 10 -3 kg

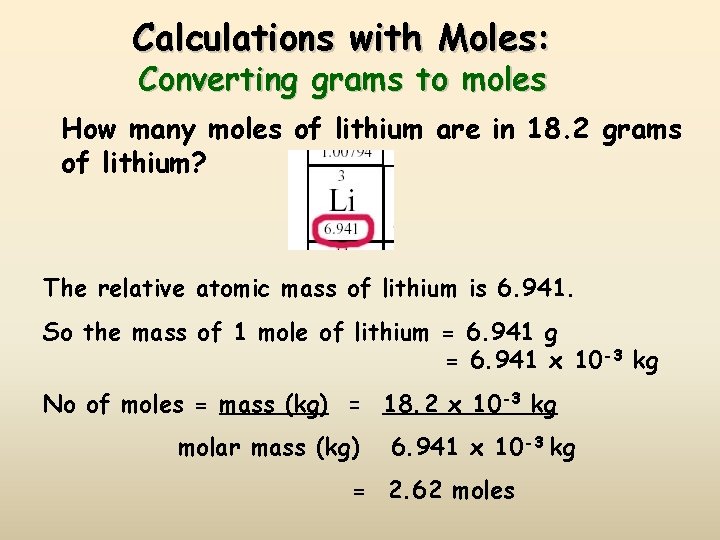
Calculations with Moles: Converting grams to moles How many moles of lithium are in 18. 2 grams of lithium? The relative atomic mass of lithium is 6. 941. So the mass of 1 mole of lithium = 6. 941 g = 6. 941 x 10 -3 kg No of moles = mass (kg) = 18. 2 x 10 -3 kg molar mass (kg) 6. 941 x 10 -3 kg = 2. 62 moles

Calculations with Moles: Using Avogadro’s Number How many atoms of lithium are in 3. 50 moles of lithium? 1 mole contains 6. 02 x 1023 atoms. So in 3. 50 moles there are 6. 02 x 1023 x 3. 50 atoms = 2. 11 x 1024 atoms

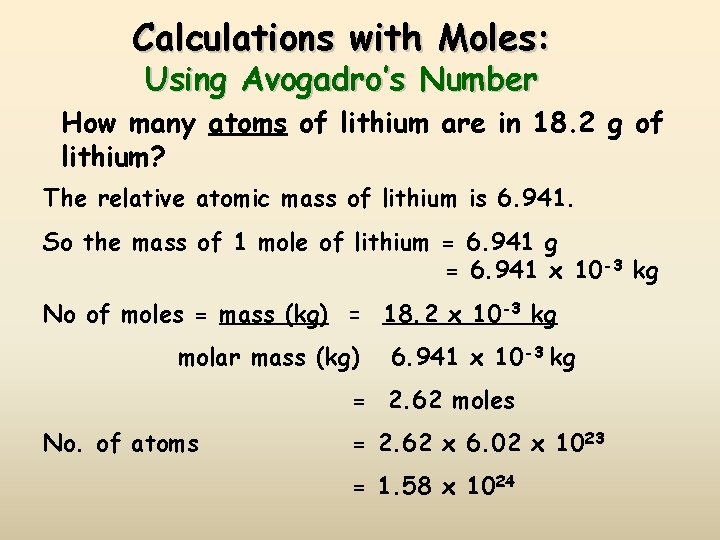
Calculations with Moles: Using Avogadro’s Number How many atoms of lithium are in 18. 2 g of lithium? The relative atomic mass of lithium is 6. 941. So the mass of 1 mole of lithium = 6. 941 g = 6. 941 x 10 -3 kg No of moles = mass (kg) = 18. 2 x 10 -3 kg molar mass (kg) 6. 941 x 10 -3 kg = 2. 62 moles No. of atoms = 2. 62 x 6. 02 x 1023 = 1. 58 x 1024












