THE MOLE 1 1 4 Explain the terms









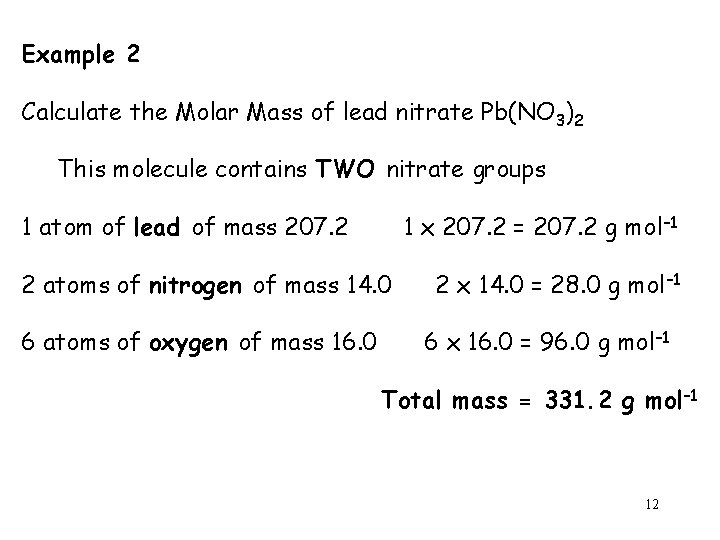




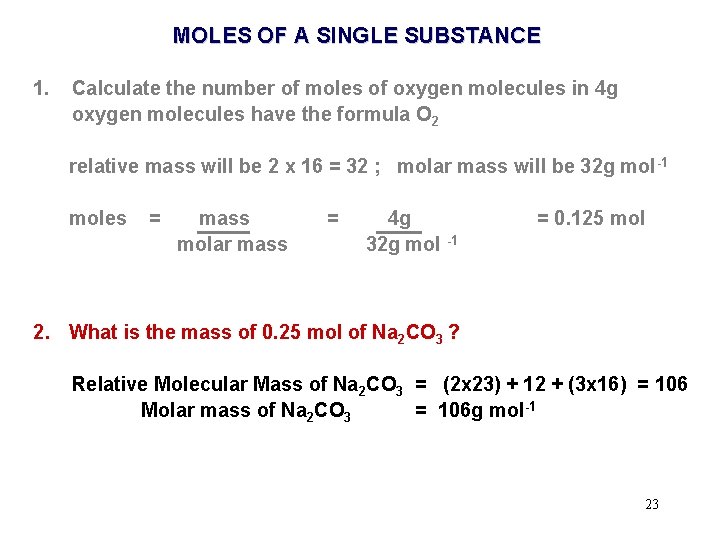
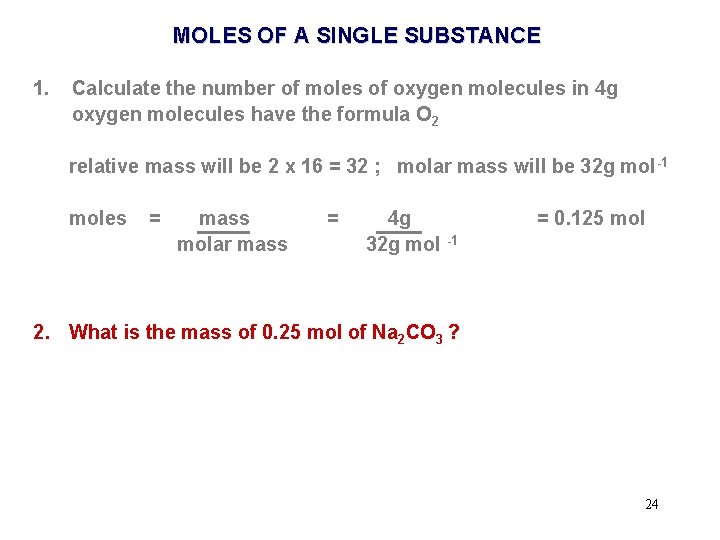
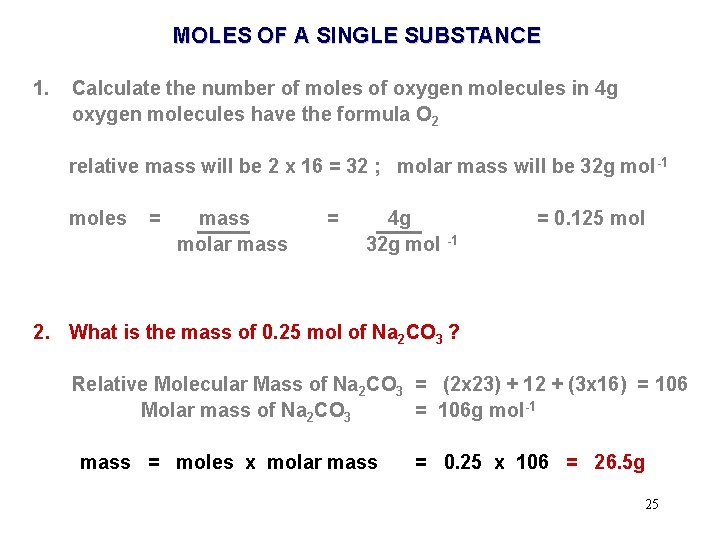
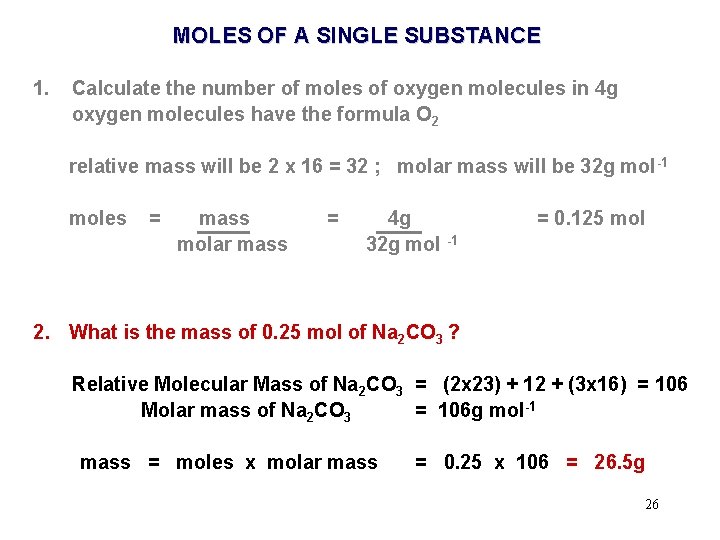






- Slides: 40

THE MOLE 1. 1. 4 • Explain the terms: amount of substance; mole; and the Avogadro constant. • Define and use the term molar mass. • Carry out calculations involving masses using the amount of substance in moles 2008 1 SPECIFICATIONS

THE MOLE Before you start it would be helpful to… • know how to balance simple equations • know how to re-arrange mathematical formulae DON’T BE LEFT IN THE DARK! 2

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144 3

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144 HOW BIG IS IT ? 60220000000000 (Approximately). . . THAT’S It is a lot easier to write it as. . . BIG !!! 6. 022 x 1023 4

THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance it is just a number, a very big number it is a way of saying a number in words, just like. . . DOZEN for 12 SCORE for 20 GROSS for 144 HOW BIG IS IT ? 60220000000000 (Approximately). . . THAT’S It is a lot easier to write it as. . . It is also known as. . . BIG !!! 6. 022 x 1023 AVOGADRO’S NUMBER 5 ! It doesn’t matter what the number is as long as everybody sticks to the same value

THE MOLE WHY USE IT ? Atoms and molecules don’t weigh much so it is easier to count large numbers of them. In fact it is easier to weigh substances. Using moles tells you. . . how many particles you get in a certain mass the mass of a certain number of particles DO I NEED TO KNOW ANYTHING ELSE ? Yes, it would help if you can balance equations AND Keep trying, you will get the idea. . . EVENTUALLY! 6

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144 7

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144 HOW BIG IS IT ? 60220000000000 (approx) - THAT’S BIG !!! It is a lot easier to write it as 6. 022 x 1023 And anyway it doesn’t matter what the number is as long as everybody sticks to the same value ! 8

THE MOLE – AN OVERVIEW WHAT IS IT? The standard unit of amount of a substance just as the standard unit of length is a METRE It is just a number, a very big number It is also a way of saying a number in words like DOZEN for 12 GROSS for 144 HOW BIG IS IT ? 60220000000000 (approx) - THAT’S BIG !!! It is a lot easier to write it as 6. 022 x 1023 And anyway it doesn’t matter what the number is as long as everybody sticks to the same value ! WHY USE IT ? Atoms and molecules don’t weigh much so it is easier to count large numbers of them. In fact it is easier to weigh substances. Using moles tells you : - how many particles you get in a certain mass the mass of a certain number of particles 9

Calculation of Molar Mass from Relative Atomic Mass data When you carry out experiments you will weigh chemicals in grams. Molar mass has the same numerical value as the. . . Relative Molecular Mass; . . . it is calculated by adding together the relative atomic masses of the elements in the molecule. The total is expressed in units of grams per mol or g mol-1. 10

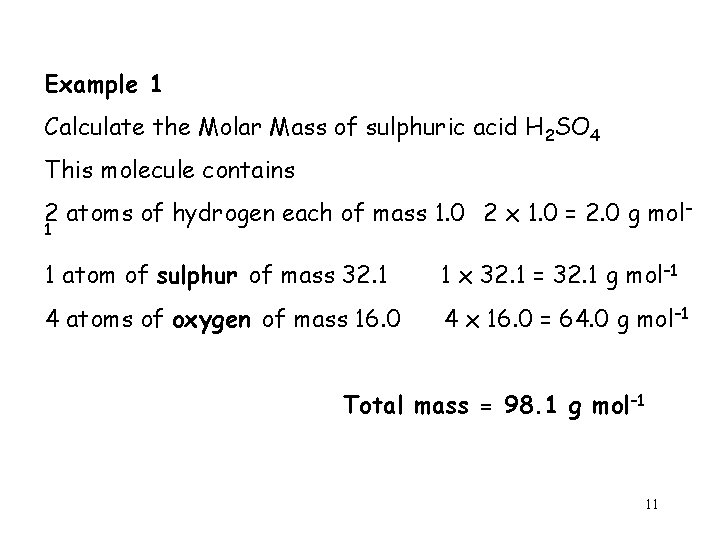
Example 1 Calculate the Molar Mass of sulphuric acid H 2 SO 4 This molecule contains 2 atoms of hydrogen each of mass 1. 0 2 x 1. 0 = 2. 0 g mol– 1 1 atom of sulphur of mass 32. 1 1 x 32. 1 = 32. 1 g mol– 1 4 atoms of oxygen of mass 16. 0 4 x 16. 0 = 64. 0 g mol– 1 Total mass = 98. 1 g mol– 1 11
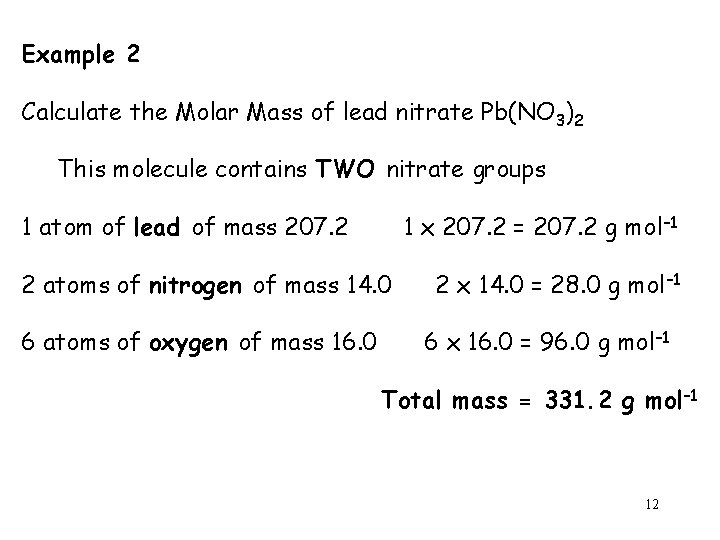
Example 2 Calculate the Molar Mass of lead nitrate Pb(NO 3)2 This molecule contains TWO nitrate groups 1 atom of lead of mass 207. 2 1 x 207. 2 = 207. 2 g mol– 1 2 atoms of nitrogen of mass 14. 0 6 atoms of oxygen of mass 16. 0 2 x 14. 0 = 28. 0 g mol– 1 6 x 16. 0 = 96. 0 g mol– 1 Total mass = 331. 2 g mol– 1 12

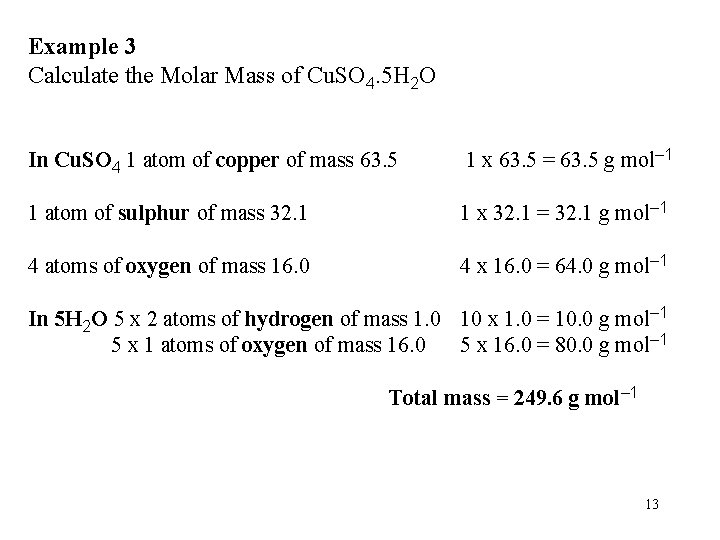
Example 3 Calculate the Molar Mass of Cu. SO 4. 5 H 2 O In Cu. SO 4 1 atom of copper of mass 63. 5 1 x 63. 5 = 63. 5 g mol– 1 1 atom of sulphur of mass 32. 1 1 x 32. 1 = 32. 1 g mol– 1 4 atoms of oxygen of mass 16. 0 4 x 16. 0 = 64. 0 g mol– 1 In 5 H 2 O 5 x 2 atoms of hydrogen of mass 1. 0 10 x 1. 0 = 10. 0 g mol– 1 5 x 1 atoms of oxygen of mass 16. 0 5 x 16. 0 = 80. 0 g mol– 1 Total mass = 249. 6 g mol– 1 13

Exercise 1. 1. 4 Molar mass odds and evens. . . 3 significant figures only ! 1, 3, 5, 7, . . . or 2, 4, 6, 8, . . . Stop at 30 !!! Now complete 31 to 60 !! Again just do - odds and evens. . . 14

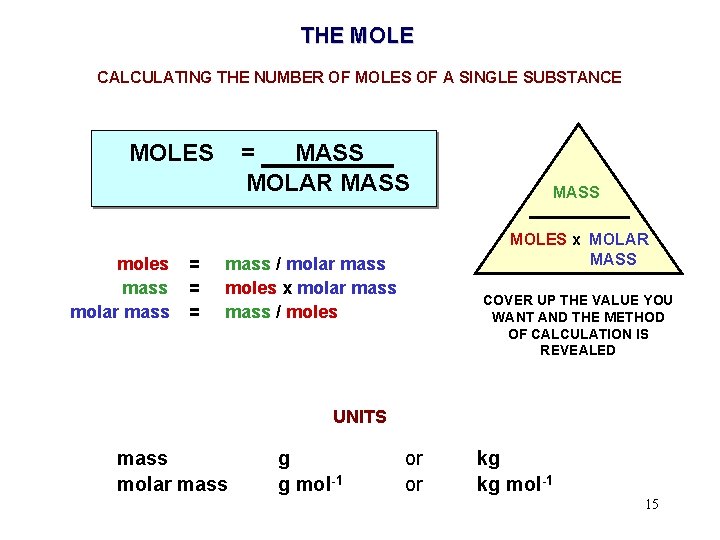
THE MOLE CALCULATING THE NUMBER OF MOLES OF A SINGLE SUBSTANCE MOLES moles mass molar mass = = MASS MOLAR MASS MOLES x MOLAR MASS mass / molar mass moles x molar mass / moles COVER UP THE VALUE YOU WANT AND THE METHOD OF CALCULATION IS REVEALED UNITS mass molar mass g g mol-1 or or kg kg mol-1 15

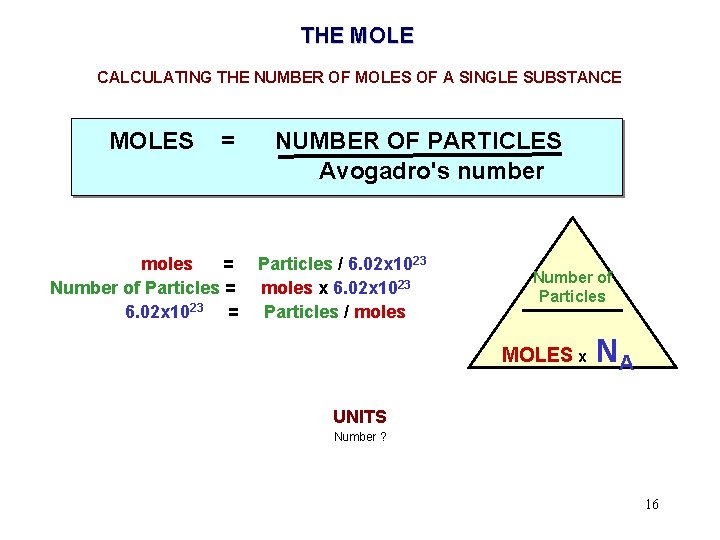
THE MOLE CALCULATING THE NUMBER OF MOLES OF A SINGLE SUBSTANCE MOLES = moles = Number of Particles = 6. 02 x 1023 = NUMBER OF PARTICLES Avogadro's number Particles / 6. 02 x 1023 moles x 6. 02 x 1023 Particles / moles Number of Particles MOLES x NA UNITS Number ? 16

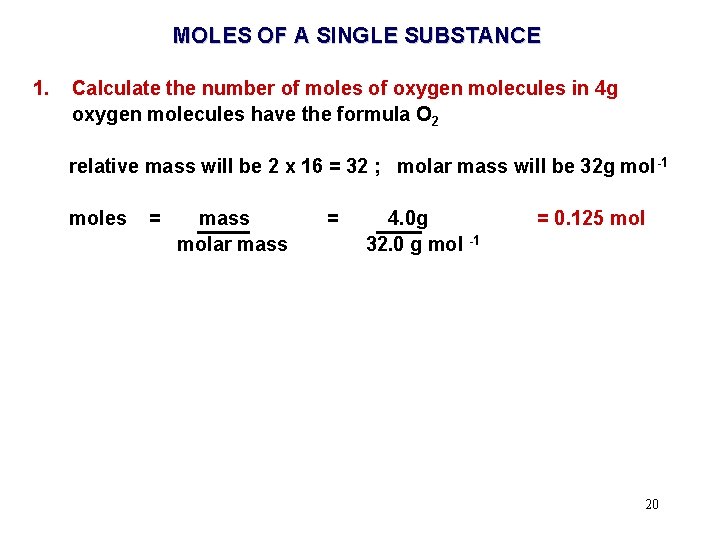
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4. 0 g 17

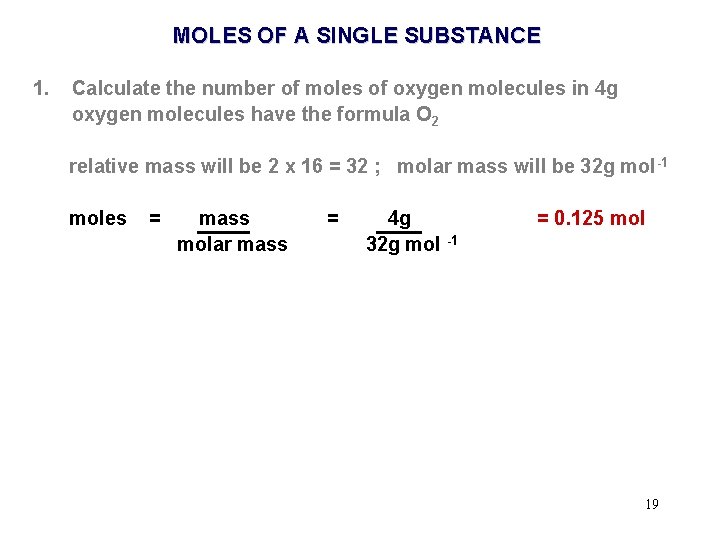
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16. 0 = 32. 0 molar mass will be 32. 0 g mol -1 18

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 19

MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4. 0 g 32. 0 g mol -1 = 0. 125 mol 20

Exercise 1. 1. 4 a Calculate the number of moles of material in a given mass of the material Again just do - odds and evens. . . 1, 3, 5, 7, . . . or 2, 4, 6, 8, . . . 3 significant figures only ! Now complete 30 ! That's only 15. . . Its easy. . 21

Exercise 1. 1. 4 Molar mass odds and evens. . . 3 significant figures only ! 1, 3, 5, 7, . . . or 2, 4, 6, 8, . . . Stop at 30 !!! Now complete 31 to 36 !! Again just do - odds and evens. . . 22
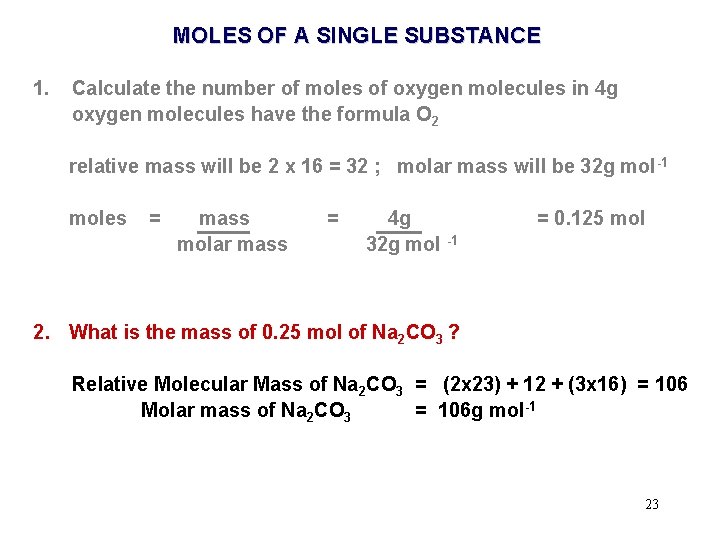
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1 23
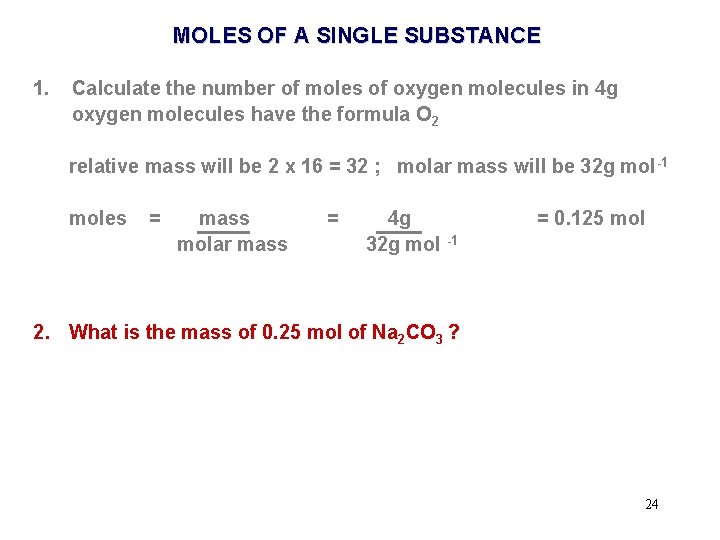
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? 24
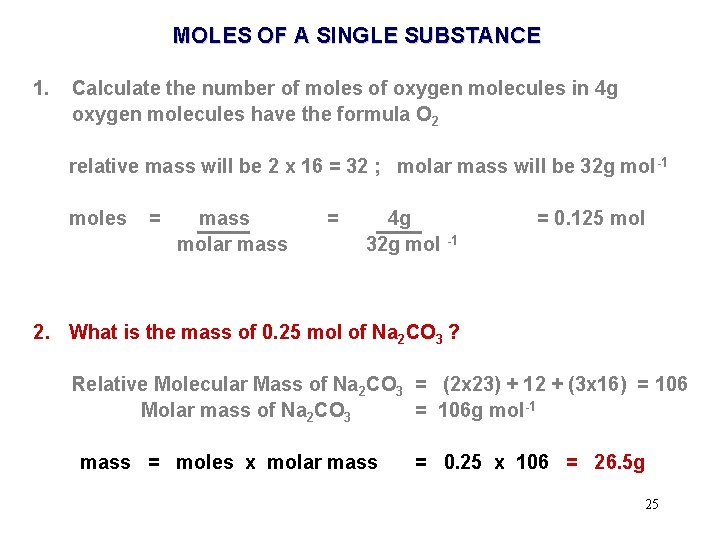
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1 mass = moles x molar mass = 0. 25 x 106 = 26. 5 g 25
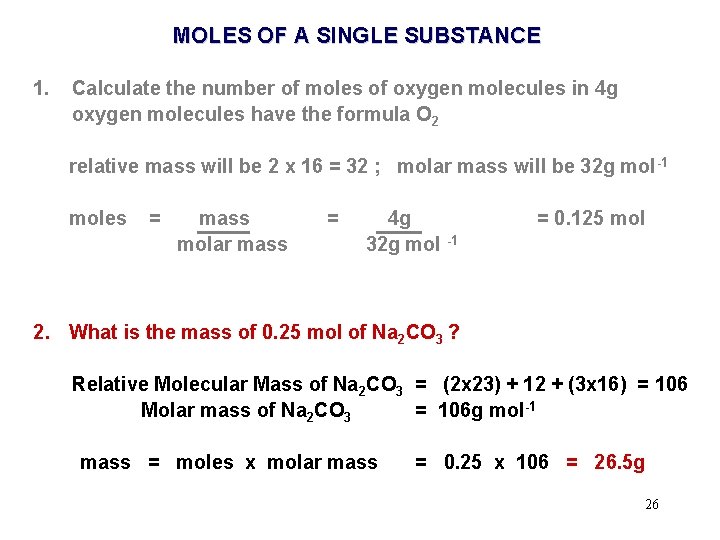
MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4 g oxygen molecules have the formula O 2 relative mass will be 2 x 16 = 32 ; molar mass will be 32 g mol -1 moles = mass molar mass = 4 g 32 g mol -1 = 0. 125 mol 2. What is the mass of 0. 25 mol of Na 2 CO 3 ? Relative Molecular Mass of Na 2 CO 3 = (2 x 23) + 12 + (3 x 16) = 106 Molar mass of Na 2 CO 3 = 106 g mol-1 mass = moles x molar mass = 0. 25 x 106 = 26. 5 g 26

Exercise 1. 1. 4 a Calculate the number of moles of material in a given mass of the material Again just do - odds and evens. . . 1, 3, 5, 7, . . . or 2, 4, 6, 8, . . . 3 significant figures only ! Now complete 50 ! That's only 25. . . Its easy. . 27

Exercise 1. 1. 4 b Calculate the mass of material in a given the number of moles of the material Again just do - odds and evens. . . 3 significant figures only ! 1, 3, 5, 7, . . . or 2, 4, 6, 8, . . . Now complete 50 ! Thats only 25. . . Its getting easier. . 28

Tasks Write out these Key definitions Amount of substance. . The Avogadro constant. . A mole. . . Molar Mass, Mr Worksheets Questions : 1, 2 29

31 Fe. Cl 3 32 Fe 2(SO 4)3 33 Pb. O 34 Pb. O 2 35 Pb 3 O 4 36 Pb(NO 3)2 37 Pb. Cl 2 38 Pb. SO 4 39 Cu. Cl 40 Cu. Cl 2 41 Cu. SO 4 42 Zn. Cl 2 43 Ag. NO 3 44 NH 4 Cl 45 (NH 4)2 SO 4 46 NH 4 VO 3 47 KCl. O 3 48 KIO 3 49 Na. Cl. O 50 Na. NO 2 51 Cu. SO 4. 5 H 2 O 52 Fe. SO 4. 7 H 2 O 53 (NH 4)2 SO 4. Fe 2(SO 4)3. 24 H 2 O 54 Na 2 S 2 O 3. 5 H 2 O 55 (COOH)2. 2 H 2 O 56 Mg. SO 4. 7 H 2 O 57 Cu(NH 3)4 SO 4. 2 H 2 O 58 CH 3 CO 2 H 59 CH 3 COCH 3 60 C 6 H 5 CO 2 H 30

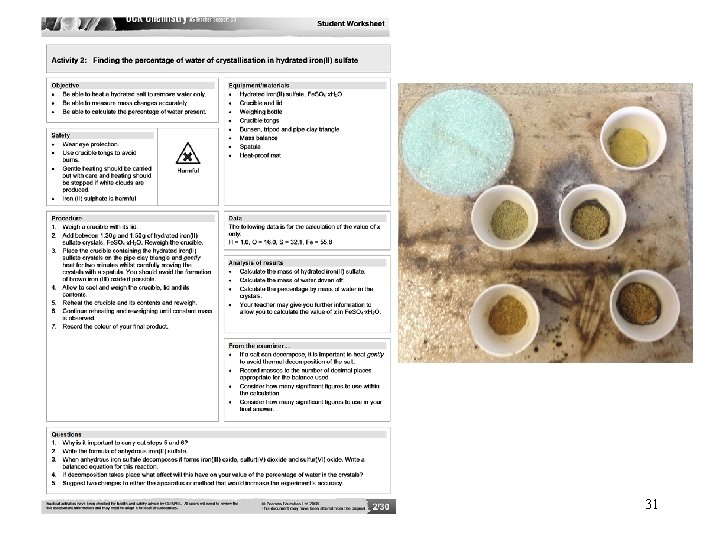
31

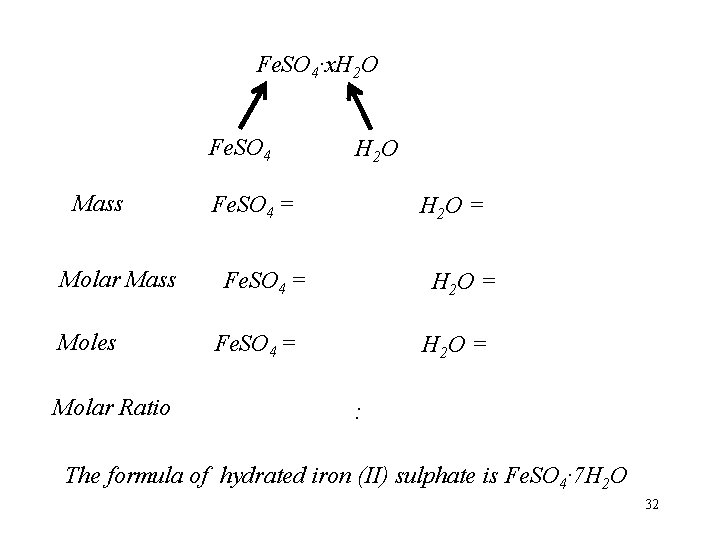
Fe. SO 4·x. H 2 O Fe. SO 4 Mass Molar Mass Moles Molar Ratio H 2 O Fe. SO 4 = H 2 O = : The formula of hydrated iron (II) sulphate is Fe. SO 4· 7 H 2 O 32

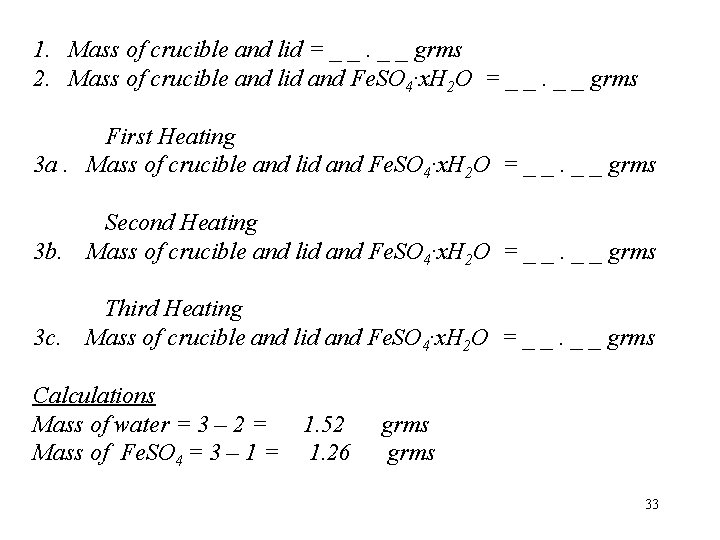
1. Mass of crucible and lid = _ _ grms 2. Mass of crucible and lid and Fe. SO 4·x. H 2 O = _ _ grms First Heating 3 a. Mass of crucible and lid and Fe. SO 4·x. H 2 O = _ _ grms 3 b. Second Heating Mass of crucible and lid and Fe. SO 4·x. H 2 O = _ _ grms 3 c. Third Heating Mass of crucible and lid and Fe. SO 4·x. H 2 O = _ _ grms Calculations Mass of water = 3 – 2 = Mass of Fe. SO 4 = 3 – 1 = 1. 52 1. 26 grms 33

1. 1. 9 Tasks Write out these Key definitions Stoichiometry is. . . Examiner tip is. . Notes Worksheets Questions : 1, 2, 3 34

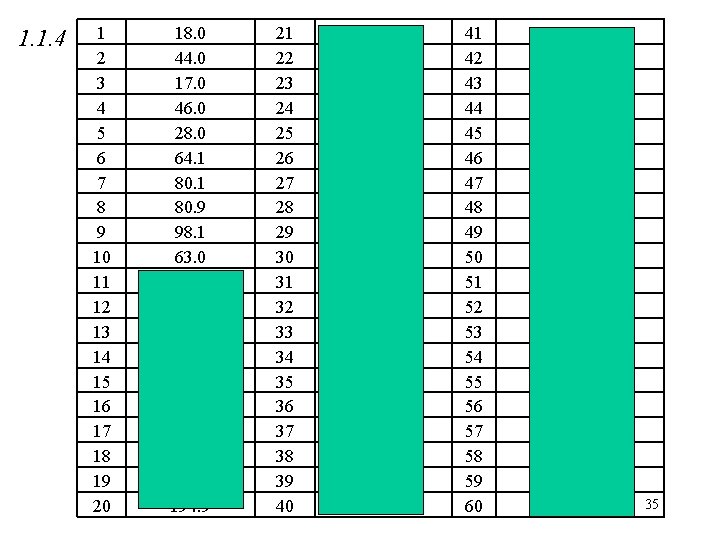
1. 1. 4 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 18. 0 44. 0 17. 0 46. 0 28. 0 64. 1 80. 9 98. 1 63. 0 58. 5 85. 0 106. 0 40. 0 142. 1 158. 0 194. 2 100. 1 166. 0 194. 9 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 111. 1 164. 1 74. 1 136. 2 208. 3 133. 5 213 342 152 127 162. 5 400 223 239 685 331 278 303 99. 0 134. 5 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 159. 5 161. 4 170 53. 5 132 117. 0 122. 5 166. 0 74. 5 69. 0 249. 5 278 964 248 126 246 263. 6 60 58 122 35

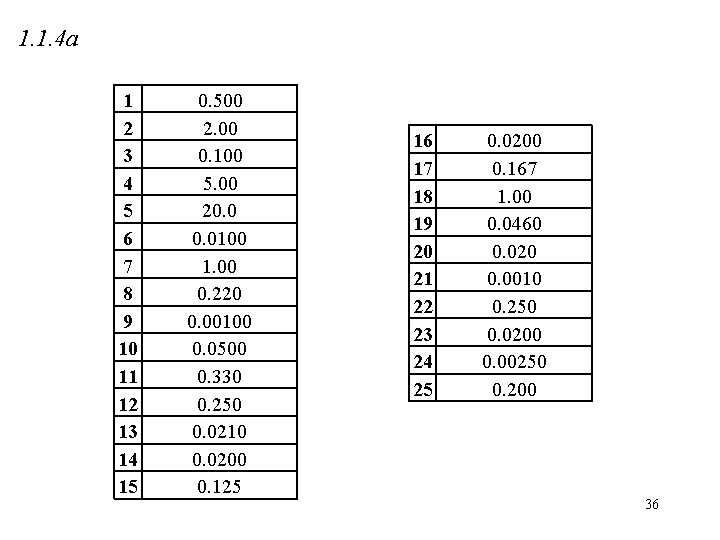
1. 1. 4 a 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 0. 500 2. 00 0. 100 5. 00 20. 0100 1. 00 0. 220 0. 00100 0. 0500 0. 330 0. 250 0. 0210 0. 0200 0. 125 16 17 18 19 20 21 22 23 24 25 0. 0200 0. 167 1. 00 0. 0460 0. 020 0. 0010 0. 250 0. 0200 0. 00250 0. 200 36

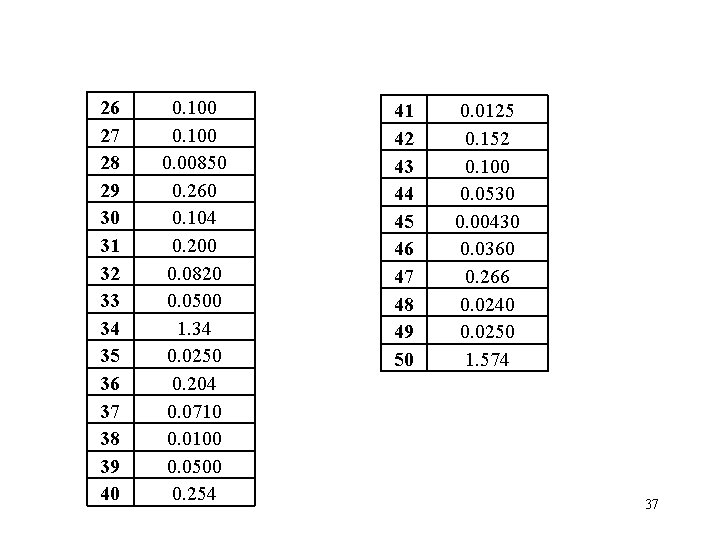
26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 0. 100 0. 00850 0. 260 0. 104 0. 200 0. 0820 0. 0500 1. 34 0. 0250 0. 204 0. 0710 0. 0100 0. 0500 0. 254 41 42 43 44 45 46 47 48 49 50 0. 0125 0. 152 0. 100 0. 0530 0. 00430 0. 0360 0. 266 0. 0240 0. 0250 1. 574 37

1. 1. 4 b 1 36 g 2 3 4 5 6 7 8 9 10 11 12 13 14 132 g 47. 6 g 23 g 33. 6 g 41. 02 g 240 g 81 g 1. 18 g 9. 45 g 26. 3 g 59. 5 g 11. 66 g 80. 0 g 15 16 17 18 19 20 21 22 23 24 25 127. 8 g 7. 9 g 34. 92 g 90 g 249 g 23. 4 g 12. 2 g 672. 4 g 0. 296 g 13. 6 g 43. 68 g 38

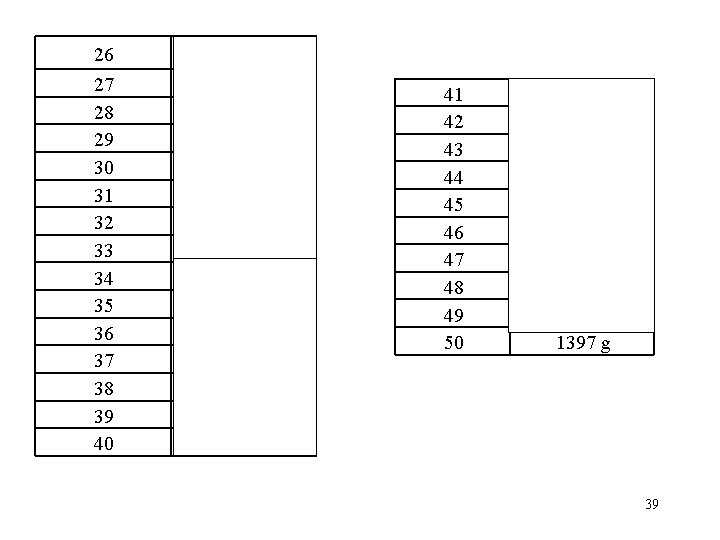
26 15. 96 g 27 28 29 30 31 32 33 34 35 36 37 38 39 40 76. 2 g 10. 03 g 17. 82 g 145. 2 g 2. 925 g 12. 25 g 21. 4 g 745 g 0. 069 g 49. 9 g 27. 8 g 4. 82 g 9. 92 g 302. 4 g 41 42 43 44 45 46 47 48 49 50 756. 5 g 39. 53 g 10. 2 g 11. 6 g 9. 76 g 4. 34 g 9. 59 g 41. 0 g 304 g 1397 g 39

40