The Metric System n A decimal system of







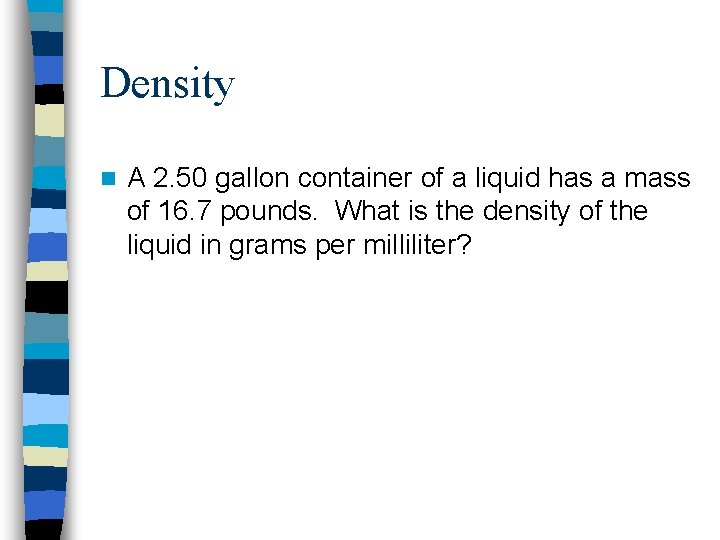
- Slides: 24

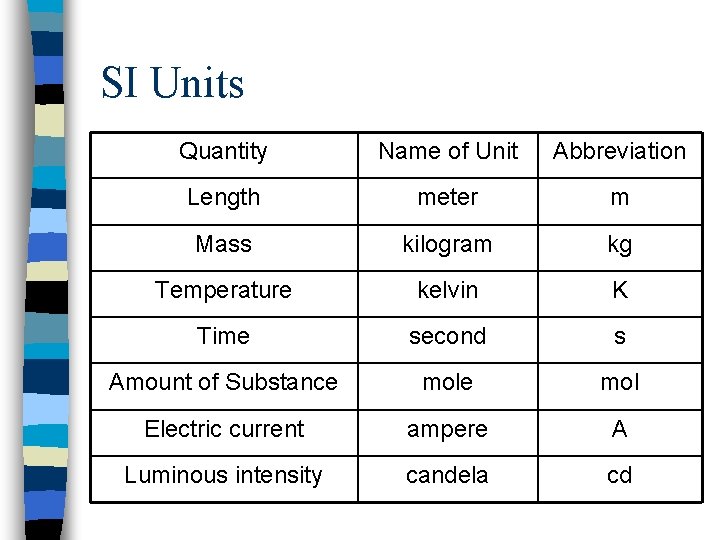
The Metric System n. A decimal system of units for measurements of length, mass, time, etc. n System International (SI) units – a superset of seven units from which all others can be derived.

SI Units Quantity Name of Unit Abbreviation Length meter m Mass kilogram kg Temperature kelvin K Time second s Amount of Substance mol Electric current ampere A Luminous intensity candela cd

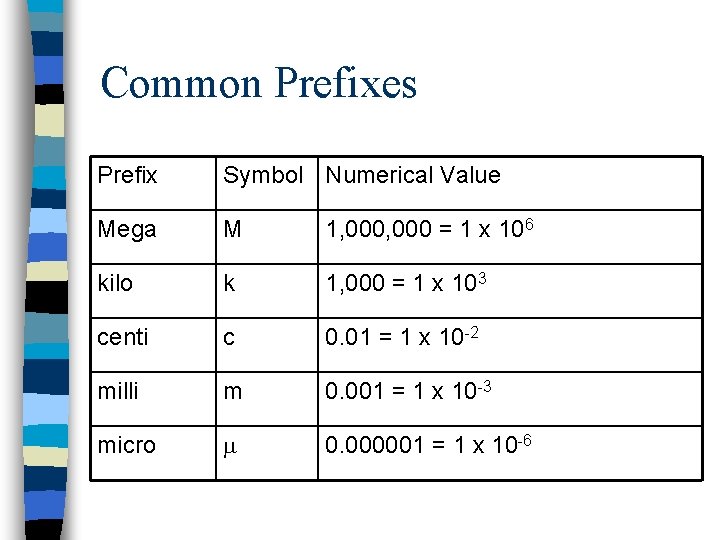
Common Prefixes Prefix Symbol Numerical Value Mega M 1, 000 = 1 x 106 kilo k 1, 000 = 1 x 103 centi c 0. 01 = 1 x 10 -2 milli m 0. 001 = 1 x 10 -3 micro m 0. 000001 = 1 x 10 -6

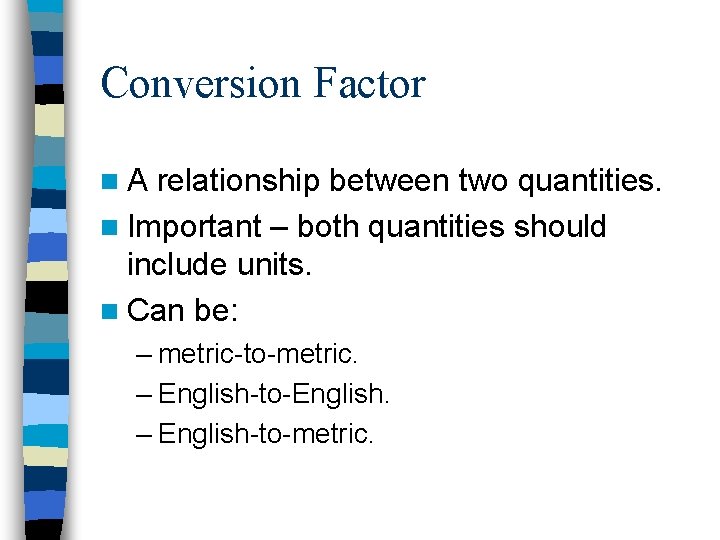
Conversion Factor n. A relationship between two quantities. n Important – both quantities should include units. n Can be: – metric-to-metric. – English-to-English. – English-to-metric.

Conversion Factor n 1 foot = 12 inches n 100 n 1 cm = 1 m inch = 2. 54 cm

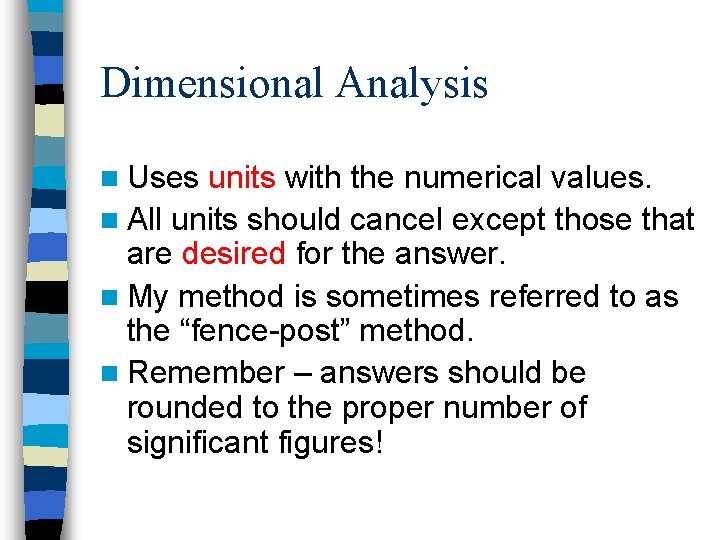
Dimensional Analysis n Uses units with the numerical values. n All units should cancel except those that are desired for the answer. n My method is sometimes referred to as the “fence-post” method. n Remember – answers should be rounded to the proper number of significant figures!

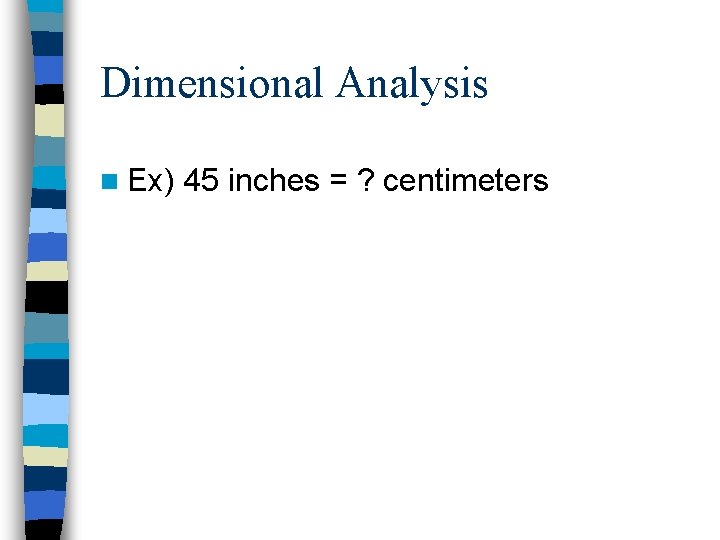
Dimensional Analysis n Ex) 45 inches = ? centimeters

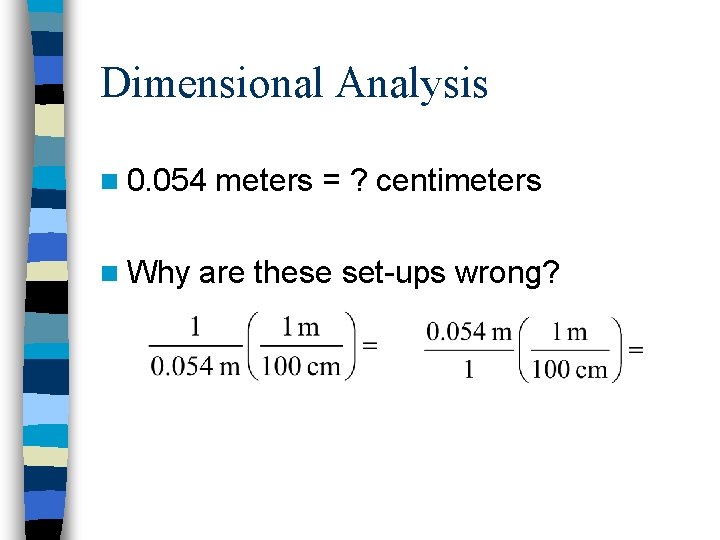
Dimensional Analysis n 0. 054 n Why meters = ? centimeters are these set-ups wrong?

Dimensional Analysis n Multi-step problems can be done either all at once or one step at a time. n Ex) 2. 50 feet = ? cm

Problem-Solving Read the problem carefully and highlight any numbers and their units. Determine what is known and what is to be solved for. 2. Plan out your course of action. What conversion factors are needed? Write them down. 1.

Problem-Solving Set-up and organize the problem in a neat and logical fashion making sure units cancel for each step. 4. Calculate your answer following proper mathematics and round to the proper number of significant digits. 5. Check your answer and work. 3.

Dimensional Analysis n Ex) 15 pints = ? L

Dimensional Analysis n 55 miles per hour = ? meters per second n How long will it take to travel 5. 0 km at this speed?

Measuring Mass and Volume n Matter = anything that occupies space and has mass. n The SI unit of mass is the kilogram. n Volume is a derived unit and is the amount of space occupied by matter. n Based on the SI system, the standard unit would be a meter x meter or cubic meter (m 3).

Measuring Mass and Volume n However, this is too large to use in practical uses. n 1 milliliter (m. L) is defined as a cube that measures 1 cm x 1 cm. n Thus, 1 m. L = 1 cm 3 or 1 cc.

Volume Conversions n. A block measures 12 inches by 6. 5 inches by 2. 5 inches. What is the volume of this block in Liters?

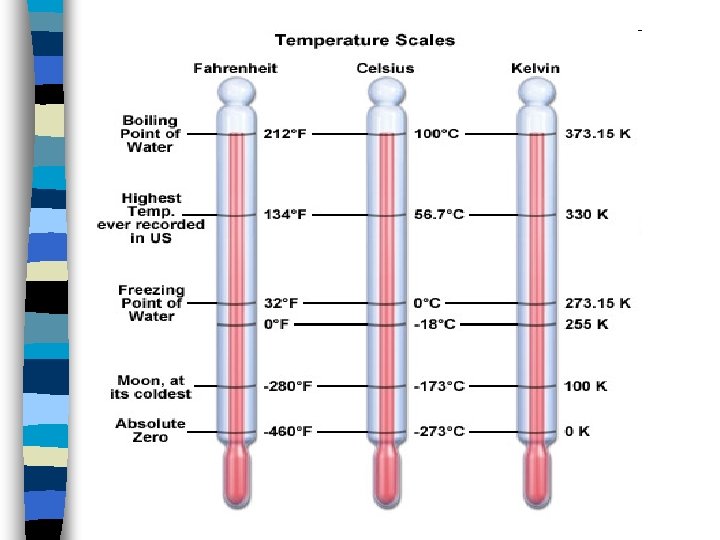
Temperature n Temperature is the measurement of the amount of heat a substance has. n Heat flows from warmer to colder objects. n SI unit is the Kelvin. n Metric unit is Celsius. n English unit is Fahrenheit.


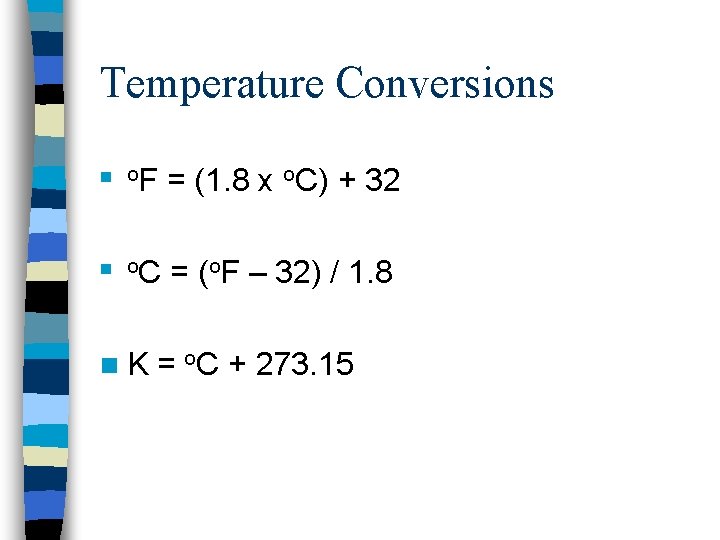
Temperature Conversions n o. F = (1. 8 x o. C) + 32 n o. C = (o. F – 32) / 1. 8 n. K = o. C + 273. 15

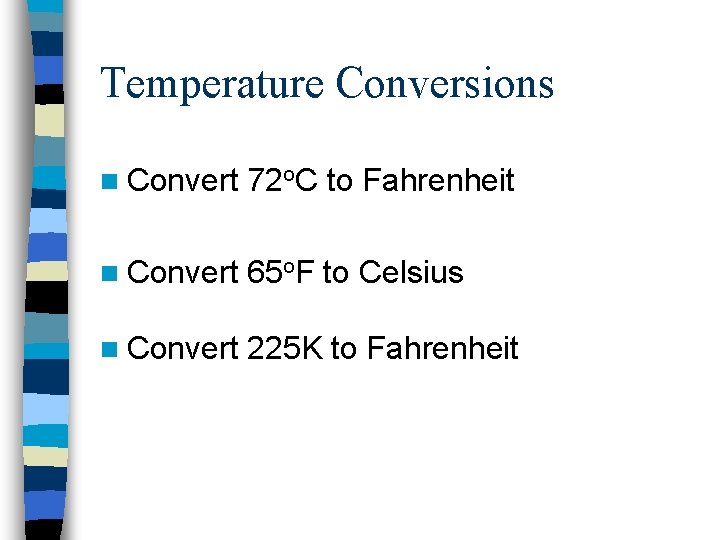
Temperature Conversions n Convert 72 o. C to Fahrenheit n Convert 65 o. F to Celsius n Convert 225 K to Fahrenheit

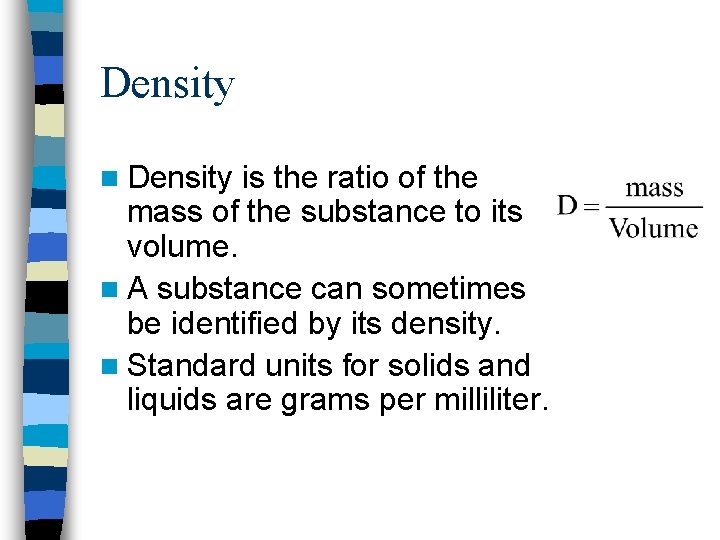
Density n Density is the ratio of the mass of the substance to its volume. n A substance can sometimes be identified by its density. n Standard units for solids and liquids are grams per milliliter.

Density n Can use the density of a substance as a conversion factor. n Density of Aluminum = 2. 70 g/m. L. n Thus, every 1 m. L of Al = 2. 70 g. n What volume would a mass of a 452 gram block of Al occupy?
Density n A 2. 50 gallon container of a liquid has a mass of 16. 7 pounds. What is the density of the liquid in grams per milliliter?
Density n The density of gold is 19. 3 g/m. L. What is the mass in grams of a gold bar measuring 6. 0 in. x 4. 0 in. x 2. 0 in. ?