The method of most probable number MPN MPN


The method of most probable number (MPN) • MPN is a statistical method to determine the bacterial population and usually is used for water or liquid samples. • MPN usually is used for the samples with lower bacterial populations. • The most common use of MPN is to determine the populations of coliform, fecal coliform, and Escherichia coli.
Indicator microorganism • Definition: indicator microorganism which can be used as an indication to monitor contamination situation of target foods. • Laboratory can not detect every pathogen but use level of indicator to monitor microbial quality of foods. • Indicator microorganisms should not be pathogens but closely related to pathogen. Thus, occurrence of indicator can be associated with pathogens.

Coliform, fecal coliforms • The most commonly used indicators are total and fecal coliforms. • Reasons: – Closed related to feces. – Exists in feces at high numbers. – Strongly survival ability in natural environment. – Fecal-oral transmission rout.

• Coliform (大腸桿菌群): microorganisms mainly live in intestinal tracks and sometimes live in natural environment. Positive results of coliform indicate possible contamination from animal feces. The 6 genera in the family of Enterobacteriaceae – Escherichia, Enterobacter, Klebsiella, Serratia, Edwardsiella, and Citrobacter. – Non-spore forming, motile, facultative aerobic, ferment lactose to produce gas

Fecal coliforms (糞便大腸桿菌群): • Fecal coliforms are capable of growth in the presence of bile salts or similar surface agents, oxidase negative, and produce acid and gas from lactose within 48 hours at 44 ± 0. 5ºC • Microorganism only live in animal intestinal tracks. Positive results of fecal coliform mean contamination of animal feces. • Fecal coliforms include the genera that originate in feces; Escherichia as well as genera that are not of fecal origin; Enterobacter, Klebsiella, and Citrobacter.
MPN • • Statistical estimate 3 -tube or 5 -tube More sensitive than plate count Key procedure – LST initial checking – BGLB confirmation for coliform – EC confirmation for fecal coliform – EMBA and EC-MUG confirmation for E. coli
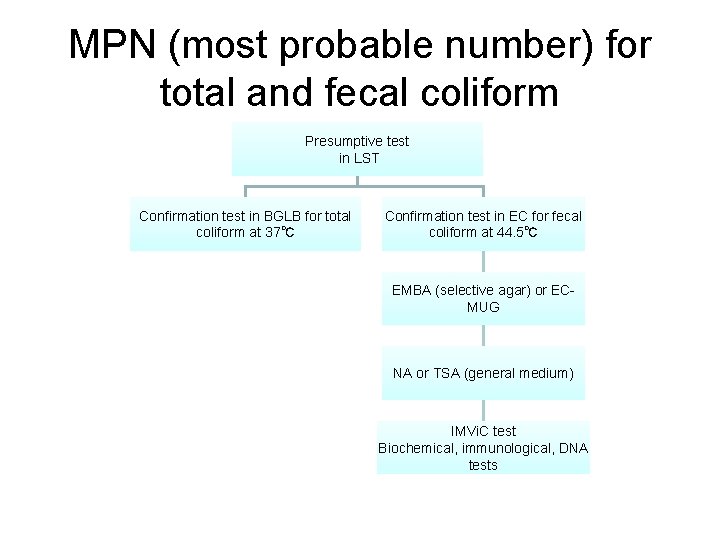
MPN (most probable number) for total and fecal coliform Presumptive test in LST Confirmation test in BGLB for total coliform at 37℃ Confirmation test in EC for fecal coliform at 44. 5℃ EMBA (selective agar) or ECMUG NA or TSA (general medium) IMVi. C test Biochemical, immunological, DNA tests

• • LST: presumptive medium BGLB: positive coliform EC: positive fecal coliform EC-MUG and EMBA: positive E. coli
MPN (most probable number) • Medium – LST (2 X and 1 X) • 2 x LST use 71. 2 g for one liter, • 1 X LST use 35. 6 g for one liter – BGLB – EC • Sample – Reverse osmosis (RO) water (negative control) – RO water inoculated with Escherichia coli and Enterobacter aerogenes (inoculated water) – Water or beverage samples (supplied by students)
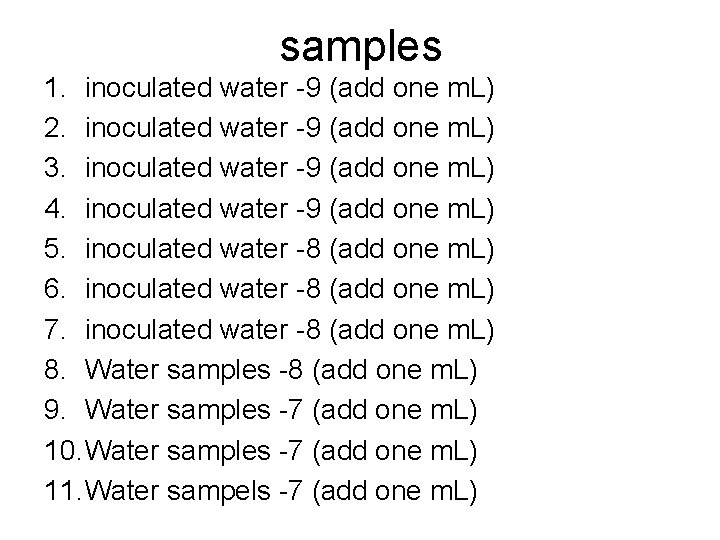
samples 1. inoculated water -9 (add one m. L) 2. inoculated water -9 (add one m. L) 3. inoculated water -9 (add one m. L) 4. inoculated water -9 (add one m. L) 5. inoculated water -8 (add one m. L) 6. inoculated water -8 (add one m. L) 7. inoculated water -8 (add one m. L) 8. Water samples -8 (add one m. L) 9. Water samples -7 (add one m. L) 10. Water samples -7 (add one m. L) 11. Water sampels -7 (add one m. L)

• Dilutions – For LST – The fist dilution (the first three tubes) use 2 X LST – Remaining LST use 1 X LST – Three dilutions per sample – Total 9 tubes per group

• BGLB • EC – Three dilutions, 9 tubes • Use fermentation tube (small tube) • Each dilution tube should has one small tube (fermentation tube) inside
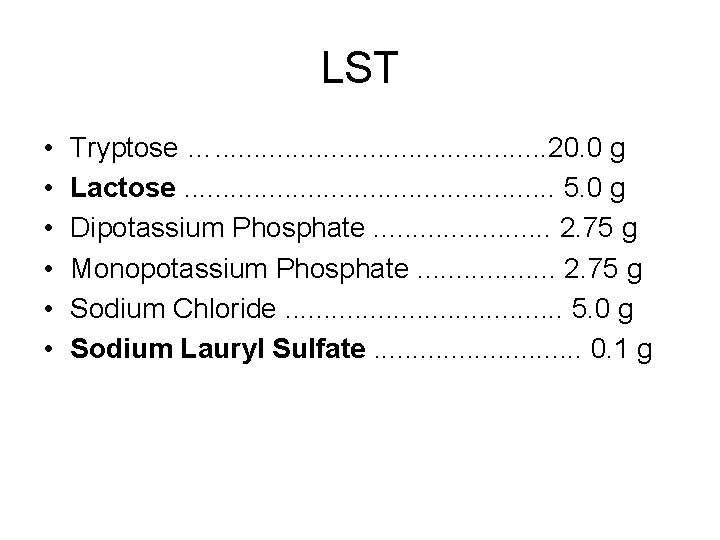
LST • • • Tryptose …. . . 20. 0 g Lactose. . . 5. 0 g Dipotassium Phosphate. . . 2. 75 g Monopotassium Phosphate. . . . 2. 75 g Sodium Chloride. . . . . 5. 0 g Sodium Lauryl Sulfate. . . . 0. 1 g

BGLB • • Peptone……. . . . 10. 0 g Oxgall. . . . . 20. 0 g Lactose. . . . . 10. 0 g Brilliant Green. . . . 13. 3 mg
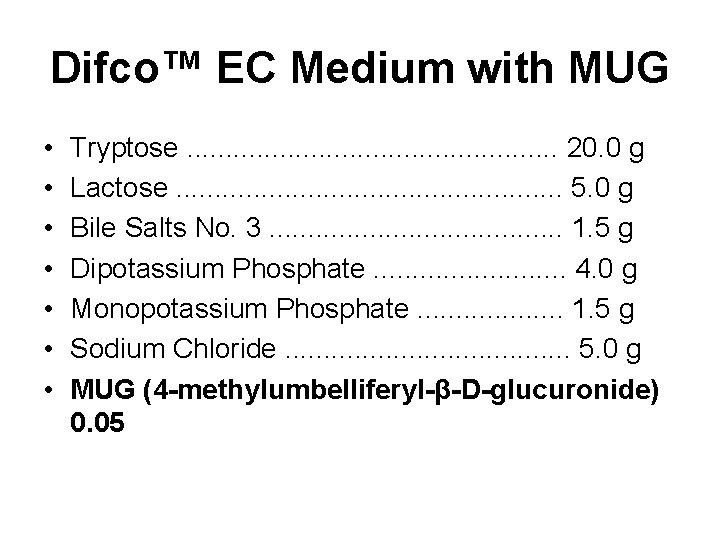
Difco™ EC Medium with MUG • • Tryptose. . . 20. 0 g Lactose. . . 5. 0 g Bile Salts No. 3. . . . . 1. 5 g Dipotassium Phosphate. . . 4. 0 g Monopotassium Phosphate. . . . . 1. 5 g Sodium Chloride. . . . . 5. 0 g MUG (4 -methylumbelliferyl-β-D-glucuronide) 0. 05
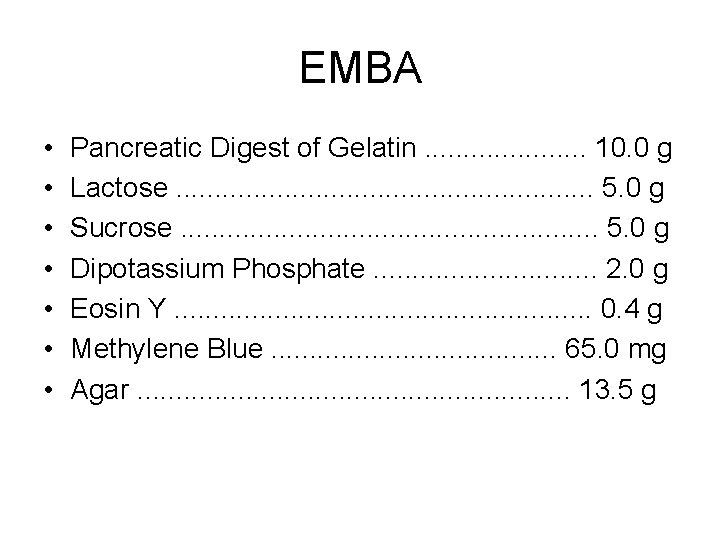
EMBA • • Pancreatic Digest of Gelatin. . . . . 10. 0 g Lactose. . . . 5. 0 g Sucrose. . . . 5. 0 g Dipotassium Phosphate. . . . 2. 0 g Eosin Y. . . . 0. 4 g Methylene Blue. . . . . 65. 0 mg Agar. . . . 13. 5 g

• The absence of β-D-glucuronidase activity in E. coli O 157: H 7 in contrast to most other E. coli. • β-D-glucuronidase cleaves 4 methylumbelliferyl-β-D-glucuronide to 4 methylumbelliferone and glucuronide. The fluorogen 4 -methylumbelliferone can be detected under a long wavelength (366 nm) UV lamp.
schedule • Monday (10/6) • MPN – Inoculated 10 m. L of water sample into the first dilution of LST medium – inoculate 1 m. L of water sample into the second dilution – Inoculate 0. 1 m. L of water sample into the third dilution – Incubate at 37 C for 48 h
• Wednesday (10/8) – Check LST medium – Transfer positive tube (turbid and with gas inside the fermentation tube) into BGLB medium and EC medium – Incubate BGLB (37 C) and EC (44. 5 C) • Thursday (10/9) – Check results of BGLB and EC tubes
• Monday (10/13) – MPN calculation – Transfer 0. 1 m. L of EC positive sample into EC -MUG tubes – Streak EC positive samples onto EMBA plate – Incubate at 37 C for 24 h – Observe on Tuesday (10/14)
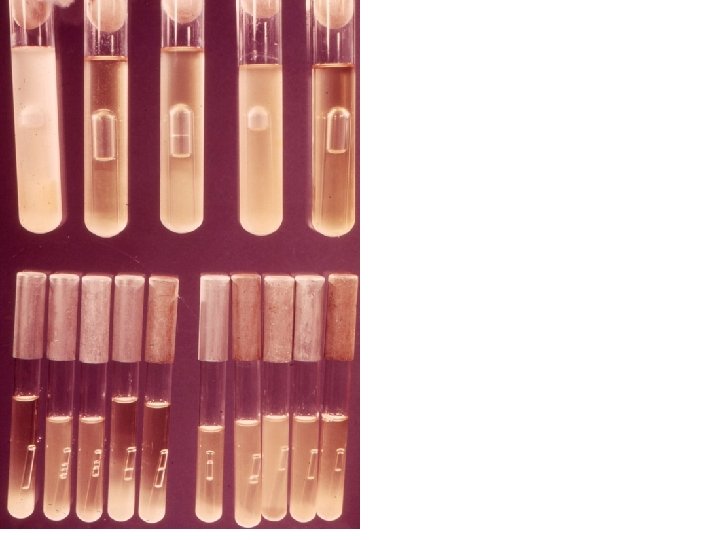
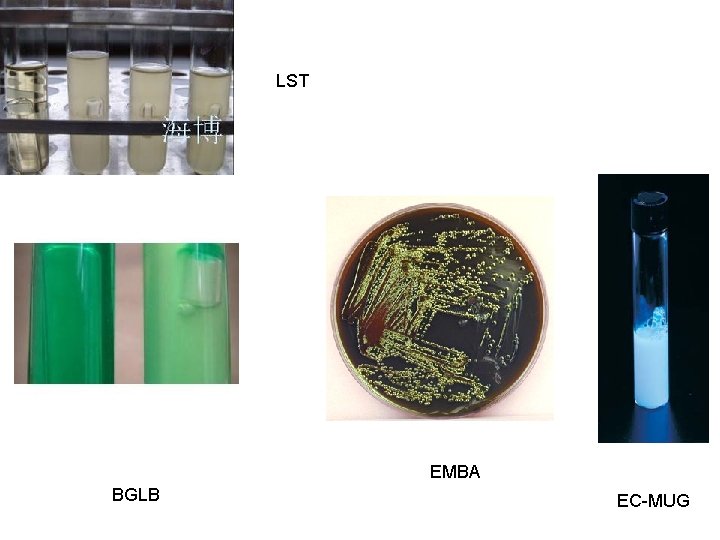
LST EMBA BGLB EC-MUG
MPN calculation • 查表 (10/1) • BGLB for coliform, EC for fecal coliform, EC-MUG and EMBA for E. coli.
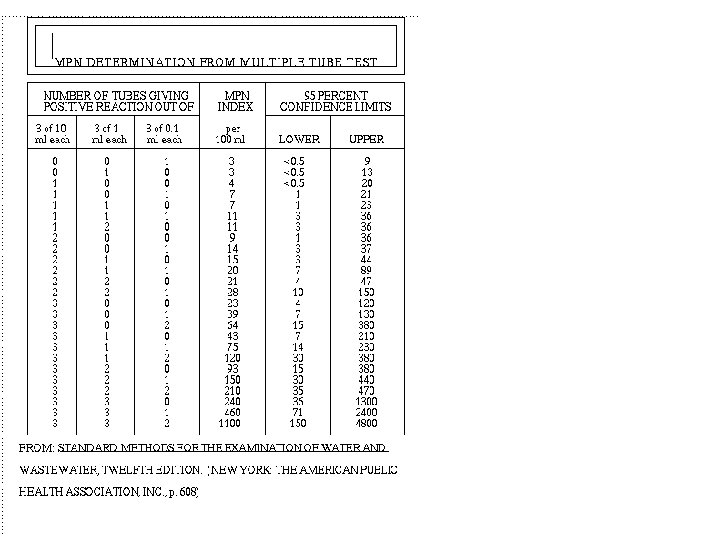
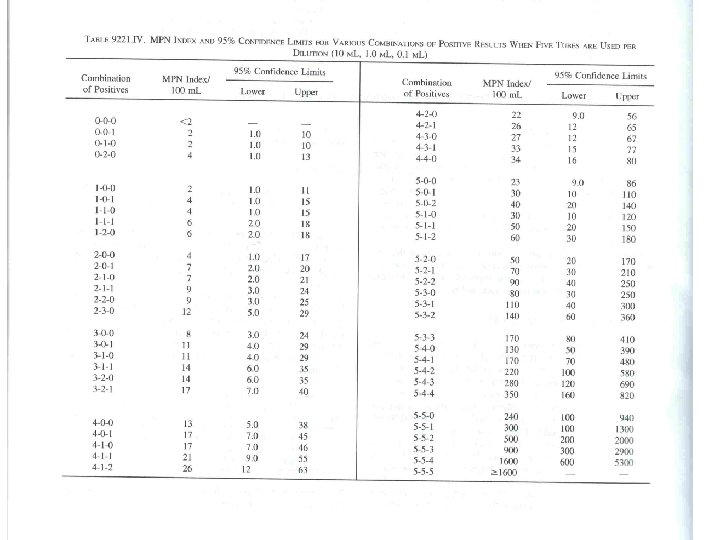
MPN table • • • Most Probable Number (最大可能數) 單位為 MPN,而非CFU Probability (機率) 測低數目菌量 取 100 m. L ,若有皆為負,則表示 3/1的100 m. L 的樣品無細菌,<3 MPN/100 m. L • 3 -tube (33. 3 m. L) and 5 -tube (55. 5 m. L)

example • Case 1: no dilution, positive number 330, MPN 240, possible range: 36 -1300. – Result: 240 MPN/100 m. L • Case 2: dilute to 10 -2, positive number 330, MPN 240, – Result: 240 x 102 = 24000 = 2. 4 x 104 MPN/100 m. L

• Usually, if a sample needs to be diluted, pour or streak plating is more accurate than MPN. • MPN method can be used for water and solid samples. • MPN method is usually used for water (liquid) samples and for Vibrio species and Staphylococcus aureus.
- Slides: 29