The Material World Classification of Elements Metals NonMetals






























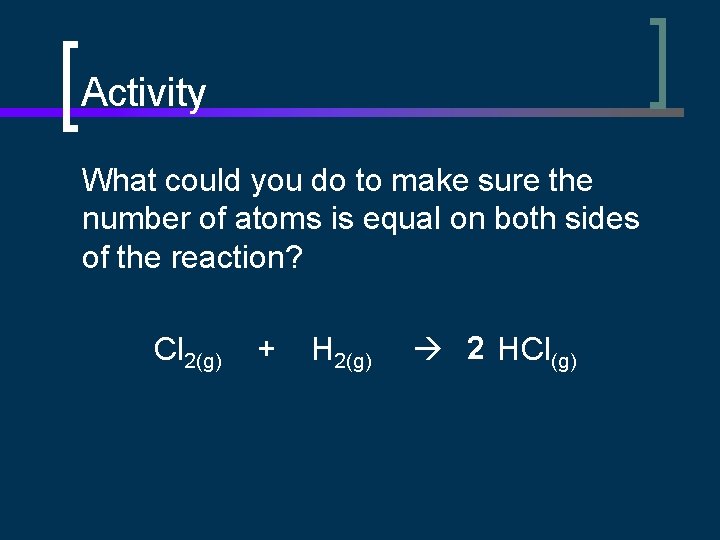

- Slides: 81

The Material World

Classification of Elements

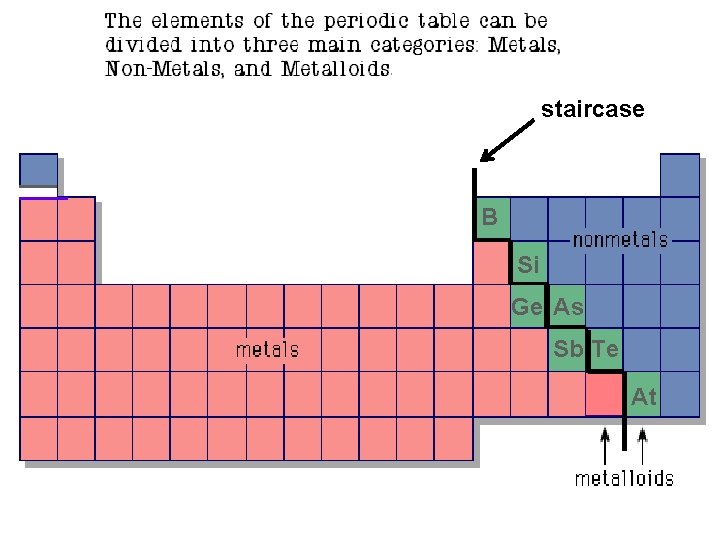
Metals, Non-Metals and Metalloids

staircase B Si Ge As Sb Te At

Properties of Metals n n n Good conductors of heat and electricity Shiny Ductile (can be stretched into thin wires) Malleable (can be pounded into thin sheets) A chemical property of metal is its reaction with water which results in corrosion

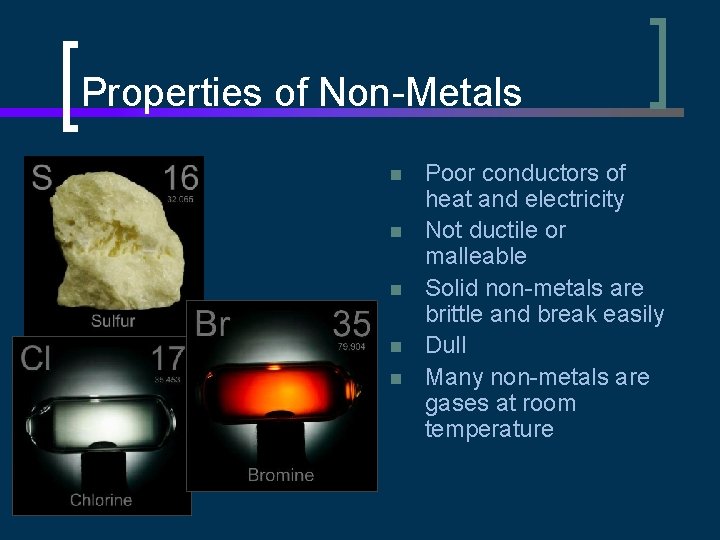
Properties of Non-Metals n n n Poor conductors of heat and electricity Not ductile or malleable Solid non-metals are brittle and break easily Dull Many non-metals are gases at room temperature

Properties of Metalloids n n n Have properties of both metals and non-metals Solids that can be shiny or dull They conduct heat and electricity better than nonmetals but not as well as metals ¡ n Used as semiconductors – materials for transistors, circuits and lasers Could be ductile and/or malleable

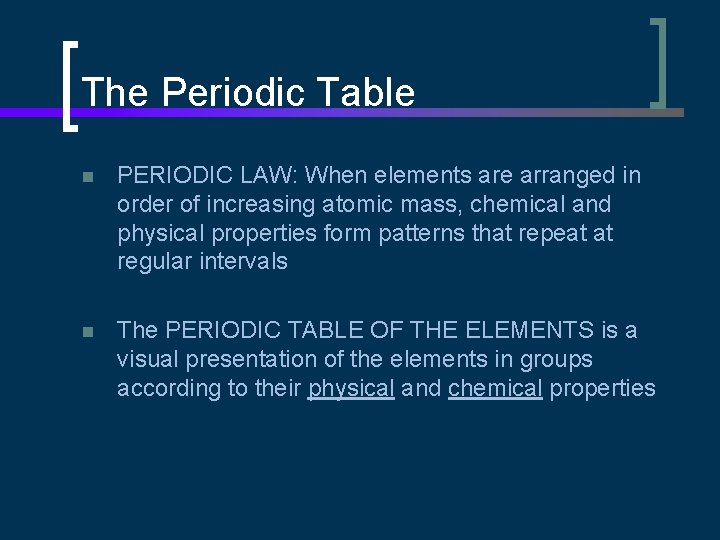
chlorine gold helium neodymium nitrogen silver oxygen hydrogen mercury niobium sodium carbon With the discovery of more elements in the early 1800`s, scientists searched for a way to organize their knowledge n

The Periodic Table n PERIODIC LAW: When elements are arranged in order of increasing atomic mass, chemical and physical properties form patterns that repeat at regular intervals n The PERIODIC TABLE OF THE ELEMENTS is a visual presentation of the elements in groups according to their physical and chemical properties

The Periodic Table n PERIODIC LAW: When elements are arranged in order of increasing atomic mass, chemical and physical properties form patterns that repeat at regular intervals n The PERIODIC TABLE OF THE ELEMENTS is a visual presentation of the elements in groups according to their physical and chemical properties

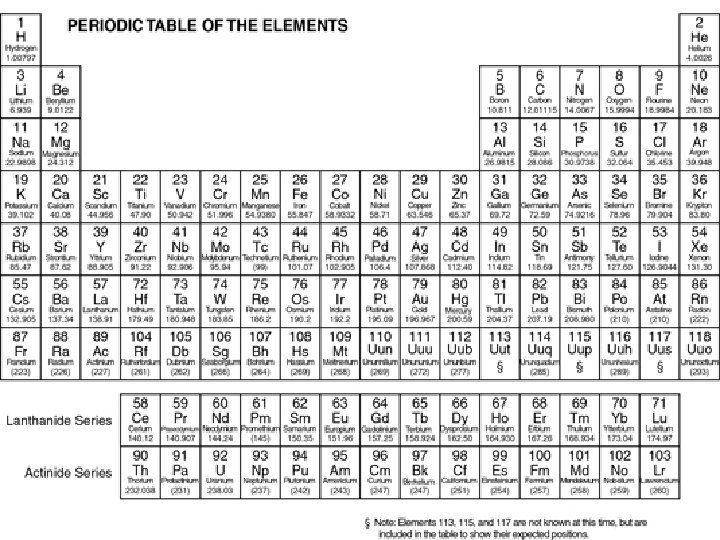
The Periodic Table number of electrons = number of protons because Elements are neutral

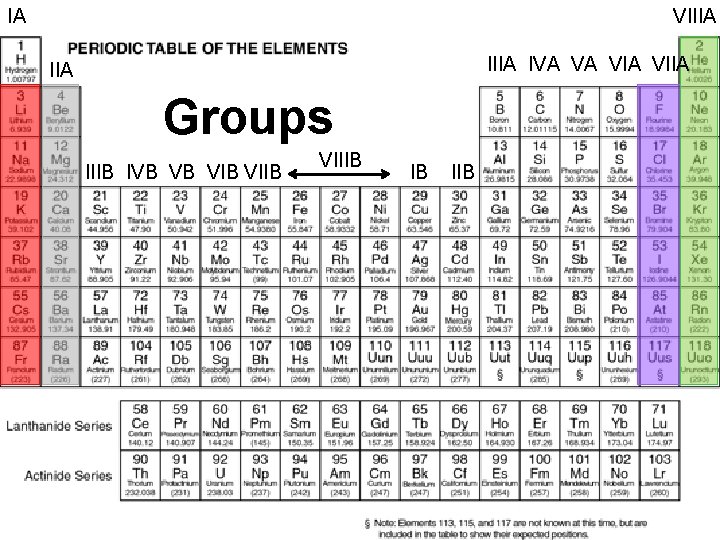
IA VIIIA IVA VA VIIA IIA Groups IIIB PERIODIC IVB VB VIB LAW: VIIB n VIIIB IB IIB are arranged in When elements order of increasing atomic mass, chemical and physical properties form patterns that repeat at regular intervals n The PERIODIC TABLE OF THE ELEMENTS is a visual presentation of the elements in groups according to their physical and chemical properties

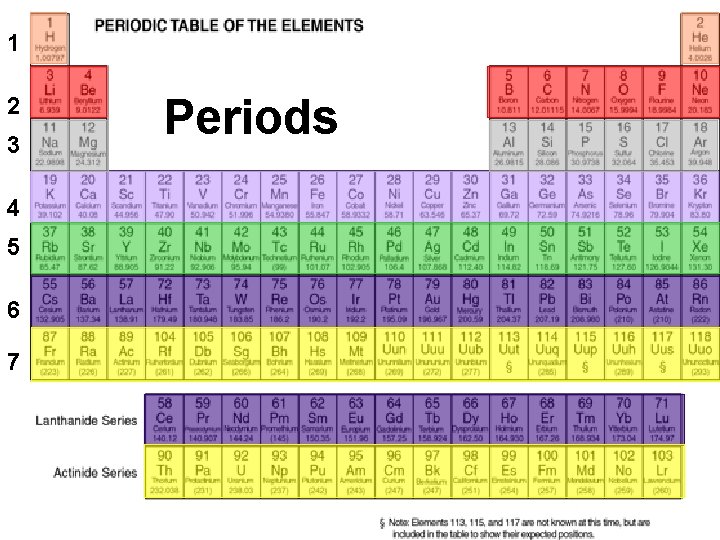
1 2 The Periodic Table Periods 3 n PERIODIC LAW: When elements are arranged in order of increasing atomic mass, chemical and physical properties form patterns that repeat at regular intervals n The PERIODIC TABLE OF THE ELEMENTS is a visual presentation of the elements in groups according to their physical and chemical properties 4 5 6 7

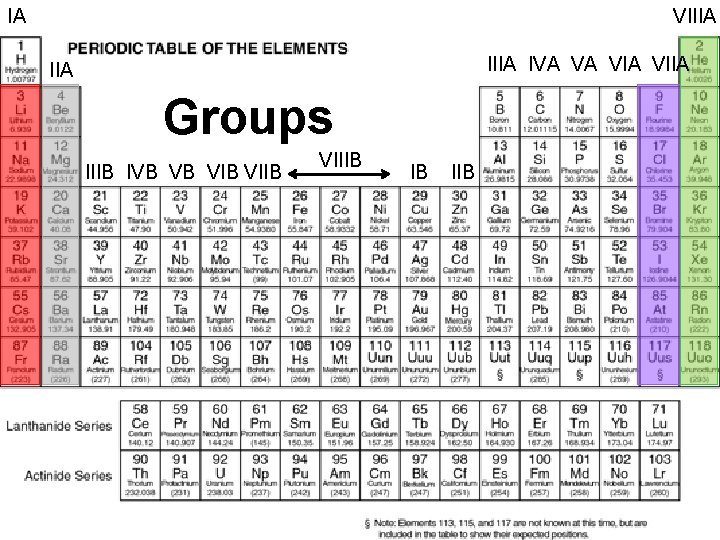
The Periodic Table: Groups n n The elements in a vertical column of the periodic table form a group or family Roman numerals and letters are used to identify different groups They have similar chemical and physical properties because they have the same number of valence electrons Valence electron: an electron in the outermost shell of an atom that is most frequently involved in chemical reactions

IA VIIIA IVA VA VIIA IIA Groups IIIB PERIODIC IVB VB VIB LAW: VIIB n VIIIB IB IIB are arranged in When elements order of increasing atomic mass, chemical and physical properties form patterns that repeat at regular intervals n The PERIODIC TABLE OF THE ELEMENTS is a visual presentation of the elements in groups according to their physical and chemical properties

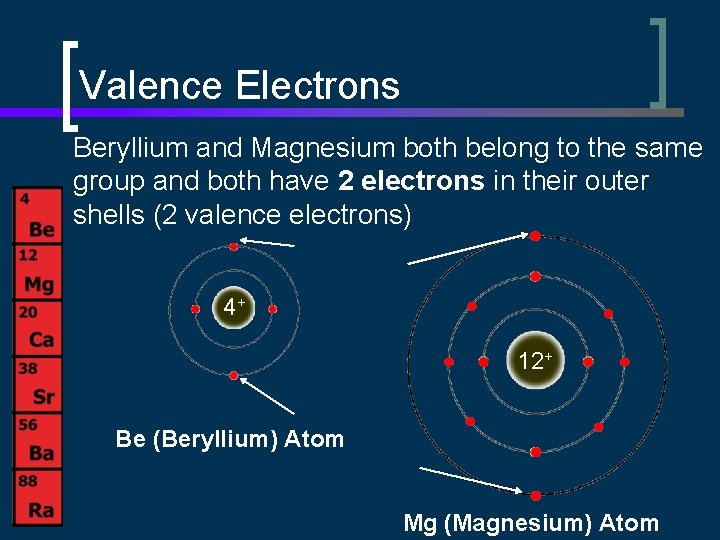
Valence Electrons Beryllium and Magnesium both belong to the same group and both have 2 electrons in their outer shells (2 valence electrons) 4+ 12+ Be (Beryllium) Atom Mg (Magnesium) Atom

Group IA n n Alkali Metals 1 valence electron Soft (can be cut by a knife) Highly reactive metals (must be stored in oil to prevent exposure to moisture in the air) ¡ ¡ n n They are not found in the elemental state in nature They appear in nature as compounds React with water to form bases DOES NOT INCLUDE HYDROGEN AT THE TOP OF THE GROUP

Group IIA n n n Alkaline Earth Metals 2 valence electrons Extremely malleable Highly reactive metals, burn easily with heat Because they are highly reactive, they are not found in the elemental state in nature ¡ They appear in nature as compounds found in rocks or earth

Group VIIA n n n Halogens 7 valence electrons Non-metals that react easily to form compounds ¡ n React with group 1 alkali metals to form salts Used as powerful disinfectants ¡ Chlorine (Cl) in swimming pools

Group VIIIA n n n Noble gases (rare gases or inert gases) 2 or 8 valence electrons Very stable, react minimally with other elements (the heavy ones only) ¡ Can be found in their elemental state in nature

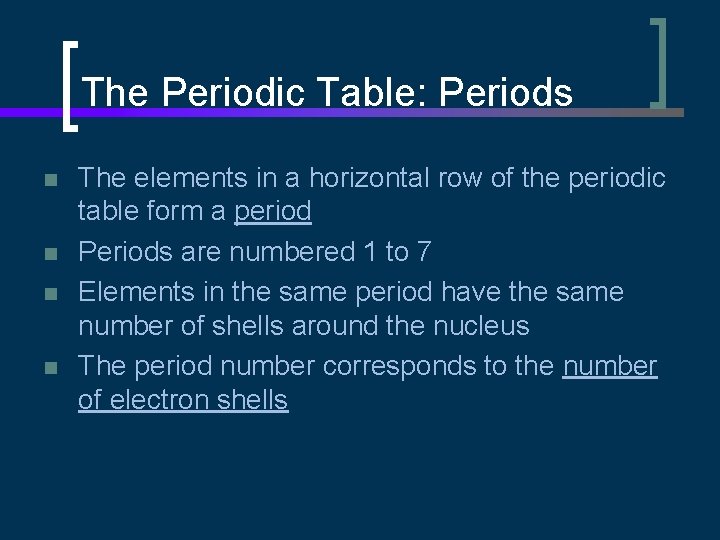
The Periodic Table: Periods n n The elements in a horizontal row of the periodic table form a period Periods are numbered 1 to 7 Elements in the same period have the same number of shells around the nucleus The period number corresponds to the number of electron shells

1 2 The Periodic Table Periods 3 n PERIODIC LAW: When elements are arranged in order of increasing atomic mass, chemical and physical properties form patterns that repeat at regular intervals n The PERIODIC TABLE OF THE ELEMENTS is a visual presentation of the elements in groups according to their physical and chemical properties 4 5 6 7

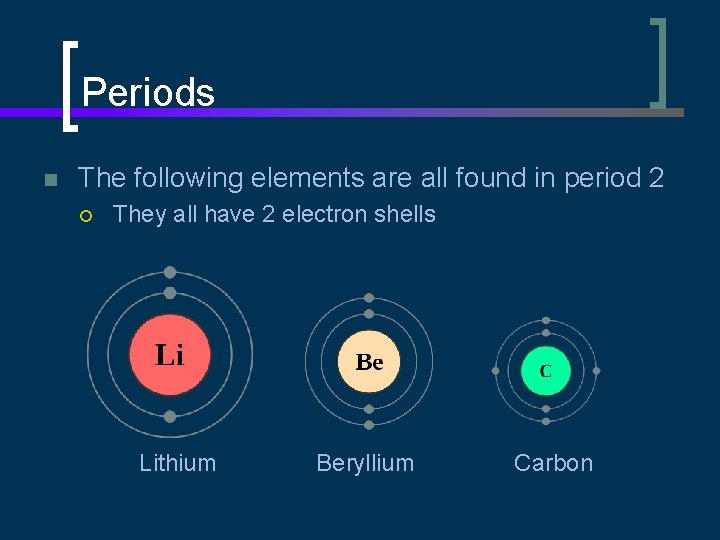
Periods n The following elements are all found in period 2 ¡ They all have 2 electron shells Lithium Beryllium Carbon

Rutherford-Bohr - n We must know the ¡ Period n ¡ the number of valence electrons Atomic number n 4+ - the number of electron shells Group n ¡ - The number of protons and electrons - Be (Beryllium) Atom

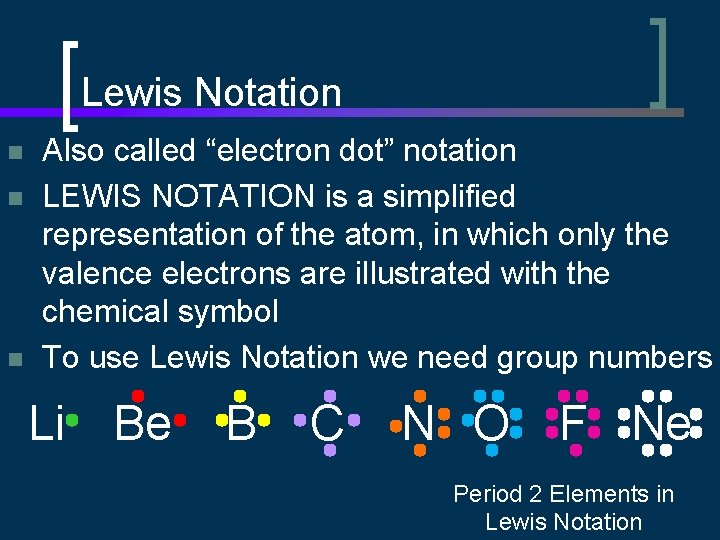
Lewis Notation n Also called “electron dot” notation LEWIS NOTATION is a simplified representation of the atom, in which only the valence electrons are illustrated with the chemical symbol To use Lewis Notation we need group numbers Li Be B C N O F Ne Period 2 Elements in Lewis Notation

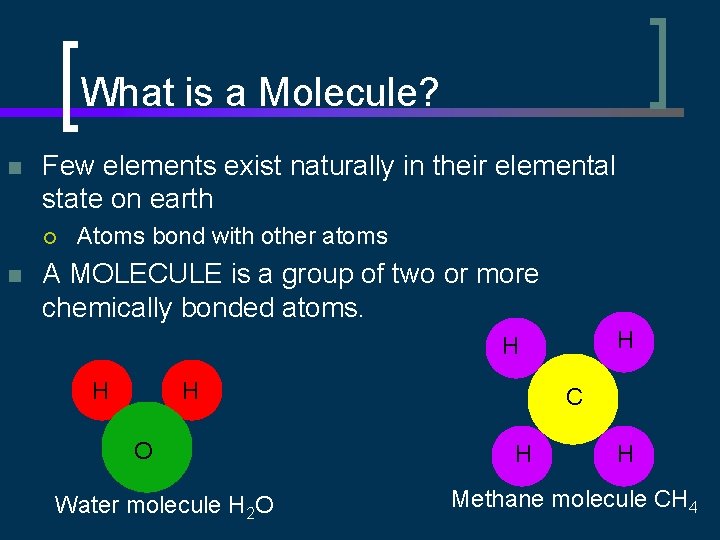
What is a Molecule? n Few elements exist naturally in their elemental state on earth ¡ n Atoms bond with other atoms A MOLECULE is a group of two or more chemically bonded atoms. H H O Water molecule H 2 O C H H Methane molecule CH 4

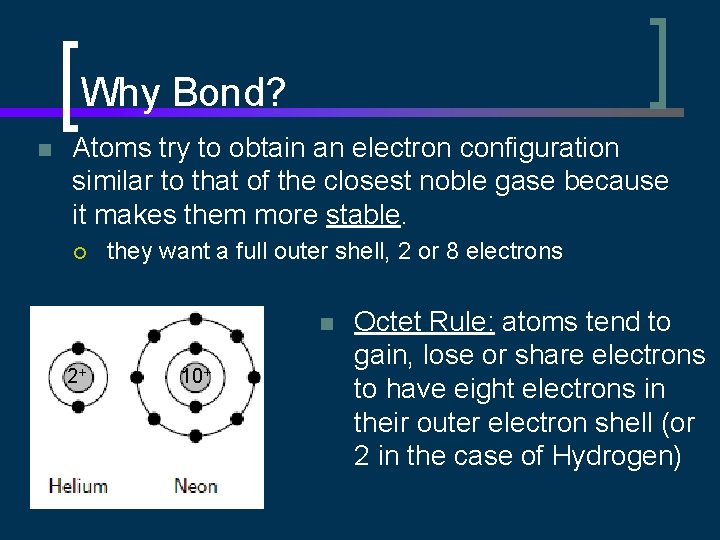
Why Bond? n Atoms try to obtain an electron configuration similar to that of the closest noble gase because it makes them more stable. ¡ they want a full outer shell, 2 or 8 electrons n 2+ 10+ Octet Rule: atoms tend to gain, lose or share electrons to have eight electrons in their outer electron shell (or 2 in the case of Hydrogen)

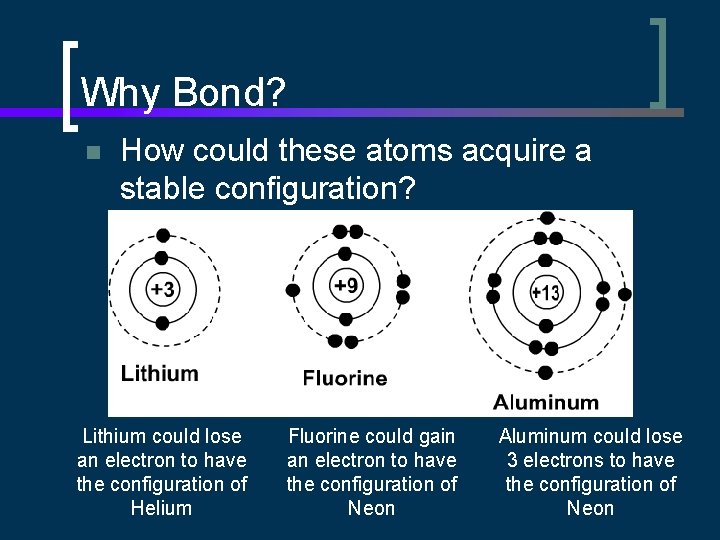
Why Bond? n How could these atoms acquire a stable configuration? Lithium could lose an electron to have the configuration of Helium Fluorine could gain an electron to have the configuration of Neon Aluminum could lose 3 electrons to have the configuration of Neon

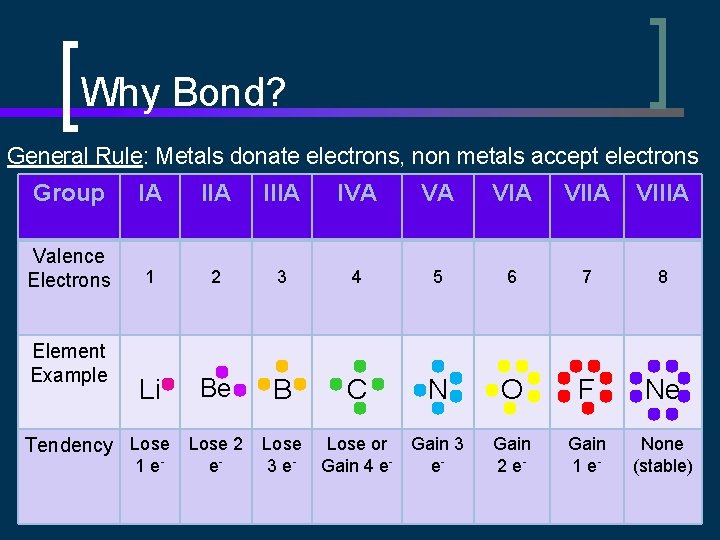
Why Bond? General Rule: Metals donate electrons, non metals accept electrons Group IA IIIA IVA VA VIIA VIIIA Valence Electrons 1 2 3 4 5 6 7 8 Li Be B C N O F Ne Lose or Gain 4 e- Gain 3 e- Gain 2 e- Gain 1 e- None (stable) Element Example Tendency Lose 2 Lose 1 e- e- 3 e-

Ions n Atoms are electrically neutral ¡ n # protons = # electrons An ION is an atom that has become electrically charged by losing or gaining one or more electrons ¡ # protons DOES NOT EQUAL # electrons

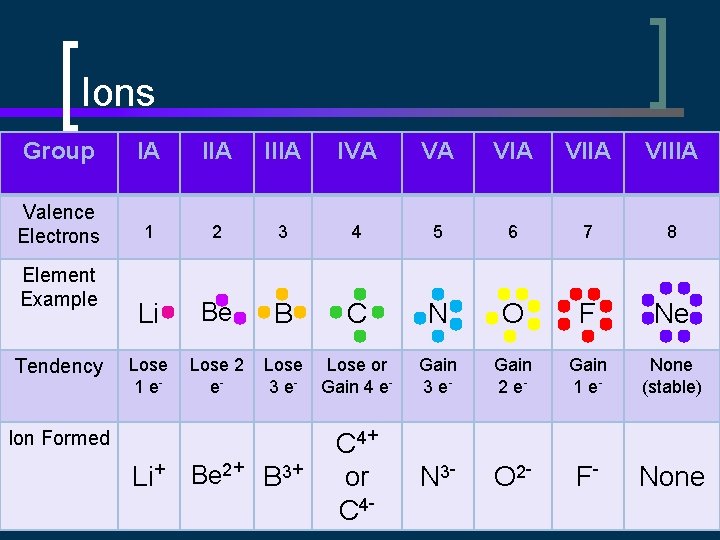
Ions Group IA IIIA IVA VA VIIA VIIIA Valence Electrons 1 2 3 4 5 6 7 8 Li Be B C N O F Ne Lose 1 e- Lose 2 e- Lose 3 e- Lose or Gain 4 e- Gain 3 e- Gain 2 e- Gain 1 e- None (stable) C 4+ or C 4 - N 3 - O 2 - F- None Element Example Tendency Ion Formed Li+ Be 2+ B 3+

Review Activity In your groups: a) Explain how the elements chlorine and sodium would each satisfy the octet rule. b) Determine the charge of the ions formed. c) Draw the Rutherford Bohr model of the ions formed.

Review Activity a) b) c) Chlorine has 7 valence electrons so it will accept an electron, sodium has 1 valence electron so it will give it away Chlorine: Sodium: +17 +11 17 protons 11 protons -18 -10 18 electrons -1 charge 10 electrons +1 charge Chlorine ion Sodium ion -1 17+ +1 11+

Properties of Solutions

Solubility of Solutions n SOLUBILITY: maximum amount of solute that can be dissolved in a volume of solvent n Solubility is affected by: ¡ The type of solute or solvent ¡ Pressure (for gas solutes) ¡ Temperature n With increasing solvent temperature, solid solutes become more soluble and gas solutes become less soluble

Concentration n n CONCENTRATION: amount of solute in a given amount of solution DISSOLUTION (addition of solute) ¡ increased concentration because more solute is added

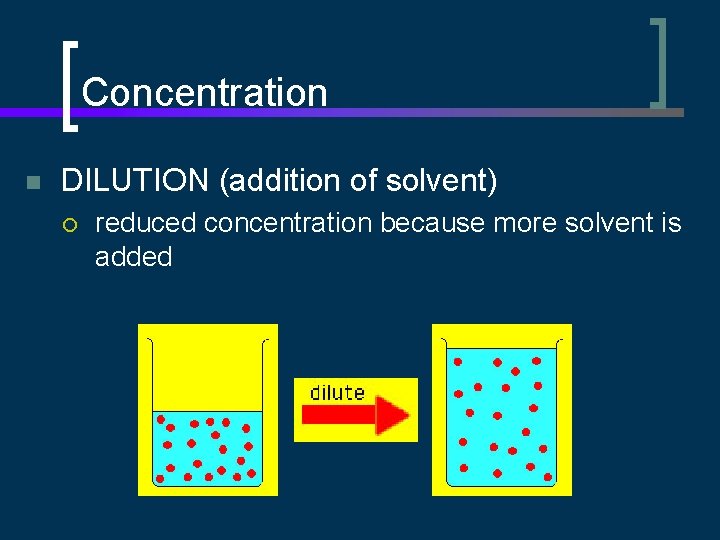
Concentration n DILUTION (addition of solvent) ¡ reduced concentration because more solvent is added

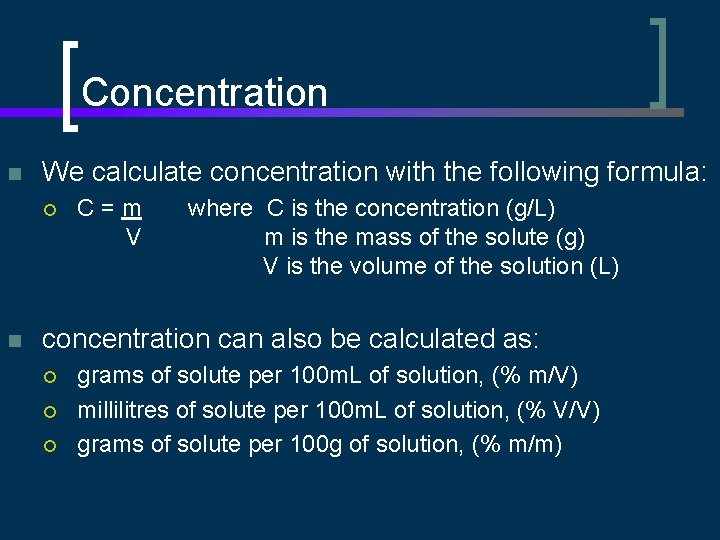
Concentration n We calculate concentration with the following formula: ¡ n C=m V where C is the concentration (g/L) m is the mass of the solute (g) V is the volume of the solution (L) concentration can also be calculated as: ¡ ¡ ¡ grams of solute per 100 m. L of solution, (% m/V) millilitres of solute per 100 m. L of solution, (% V/V) grams of solute per 100 g of solution, (% m/m)

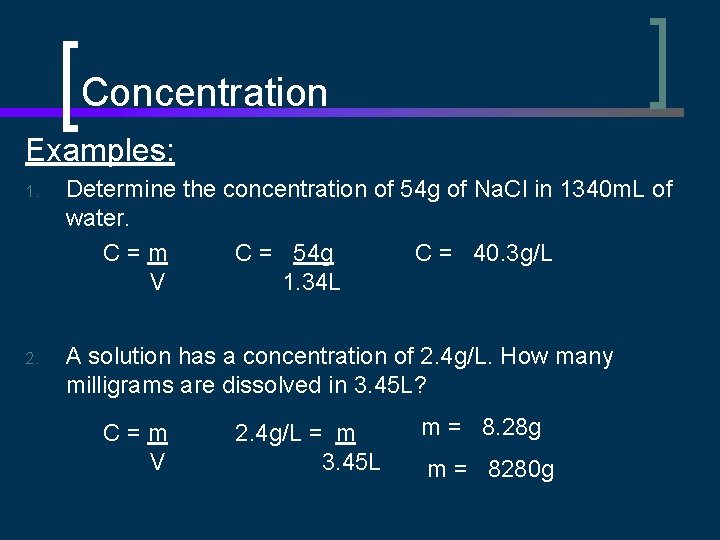
Concentration Examples: 1. Determine the concentration of 54 g of Na. Cl in 1340 m. L of water. C = 40. 3 g/L C=m C = 54 g V 1. 34 L 2. A solution has a concentration of 2. 4 g/L. How many milligrams are dissolved in 3. 45 L? C=m V 2. 4 g/L = m 3. 45 L m = 8. 28 g m = 8280 g

Concentration Examples: 3. Brine is a solution of sodium chloride at 18% m/V. How much salt is required to prepare 250 m. L of brine? 18% m/V = 18 g/100 m. L 18 g = x 100 m. L 250 m. L x = 45 g

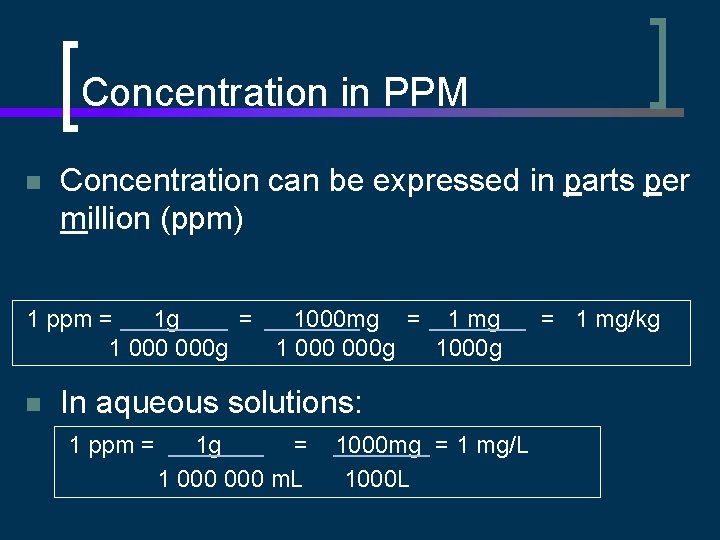
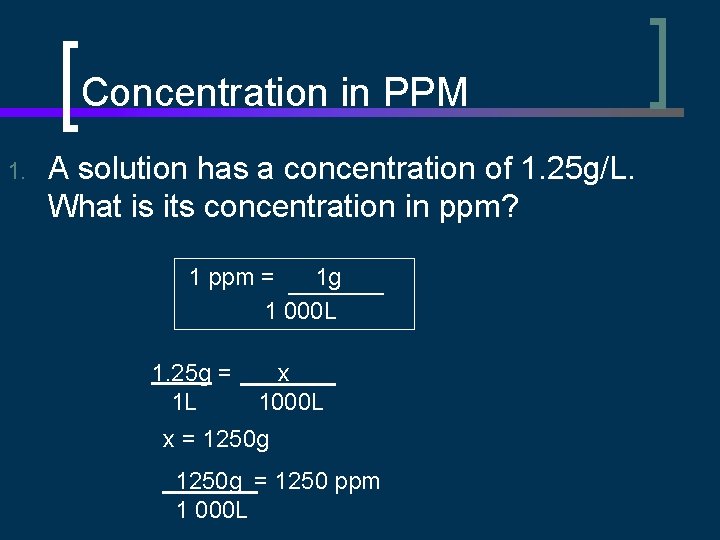
Concentration in PPM n Concentration can be expressed in parts per million (ppm) 1 ppm = 1 g = 1000 mg = 1 mg 1 000 000 g 1000 g n In aqueous solutions: 1 ppm = 1 g = 1 000 m. L 1000 mg = 1 mg/L 1000 L = 1 mg/kg

Concentration in PPM 1. A solution has a concentration of 1. 25 g/L. What is its concentration in ppm? 1 ppm = 1 g 1 000 L 1. 25 g = 1 L x 1000 L x = 1250 g = 1250 ppm 1 000 L

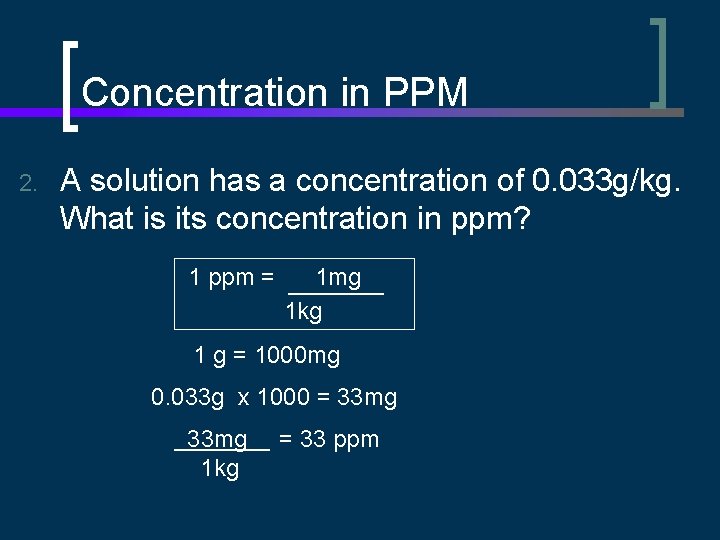
Concentration in PPM 2. A solution has a concentration of 0. 033 g/kg. What is its concentration in ppm? 1 ppm = 1 mg 1 kg 1 g = 1000 mg 0. 033 g x 1000 = 33 mg 1 kg = 33 ppm

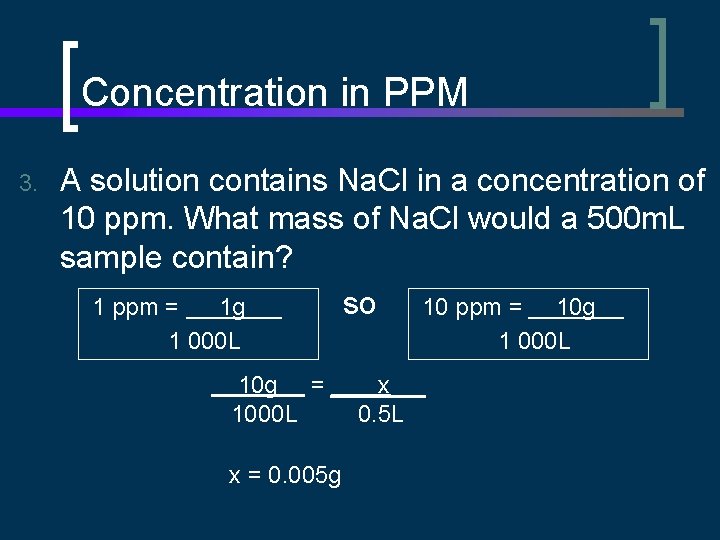
Concentration in PPM 3. A solution contains Na. Cl in a concentration of 10 ppm. What mass of Na. Cl would a 500 m. L sample contain? so 10 ppm = 10 g 1 ppm = 1 g 1 000 L 10 g = 1000 L x = 0. 005 g 1 000 L x 0. 5 L

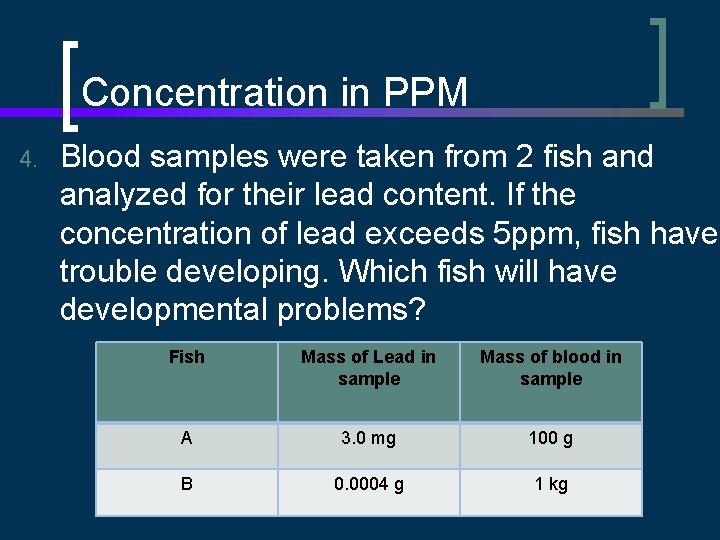
Concentration in PPM 4. Blood samples were taken from 2 fish and analyzed for their lead content. If the concentration of lead exceeds 5 ppm, fish have trouble developing. Which fish will have developmental problems? Fish Mass of Lead in sample Mass of blood in sample A 3. 0 mg 100 g B 0. 0004 g 1 kg

Concentration in PPM Fish A 1 ppm = 3. 0 mg 0. 1 kg Fish B 1 mg 1 kg = 30 ppm 1 ppm = 1 mg 1 kg 0. 0004 g = 0. 4 mg 1 kg = 0. 4 ppm Fish A will have developmental problems

Electrical Conductivity

Electrical Conductivity n ELECTROLYTE: substance that allows an electric current to flow when dissolved in water ¡ n NON ELECTROLYTES dissolve in water but don`t conduct electricity ELECTRICAL CONDUCTIVITY: a measure of a solution`s ability to allow an electric current to flow through it.

Electrolytic Dissociation ELECTROLYTIC DISSOCIATION is the separation of a dissolved compound into two ions of opposite charge. Example: n Sodium Chloride H 2 O Na. Cl(s) Na+(aq) + Cl-(aq) Silver Nitrate H 2 O Ag. NO 3(s) Ag+(aq) + NO 3 -(aq)

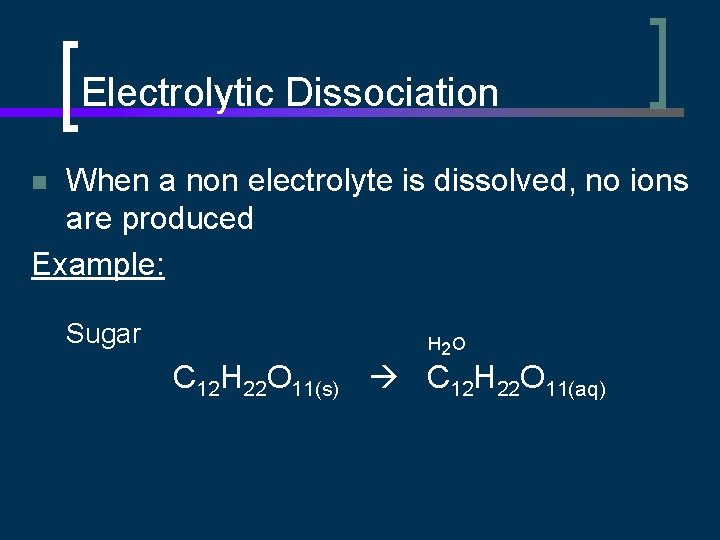
Electrolytic Dissociation When a non electrolyte is dissolved, no ions are produced Example: n Sugar H 2 O C 12 H 22 O 11(s) C 12 H 22 O 11(aq)

Types of Electrolytes n Three different types of electrolytic solutions: ¡ Acidic ¡ Basic (Alkaline) ¡ Salt

Acids n n They taste sour Change the color of indicators - Blue litmus turns to red

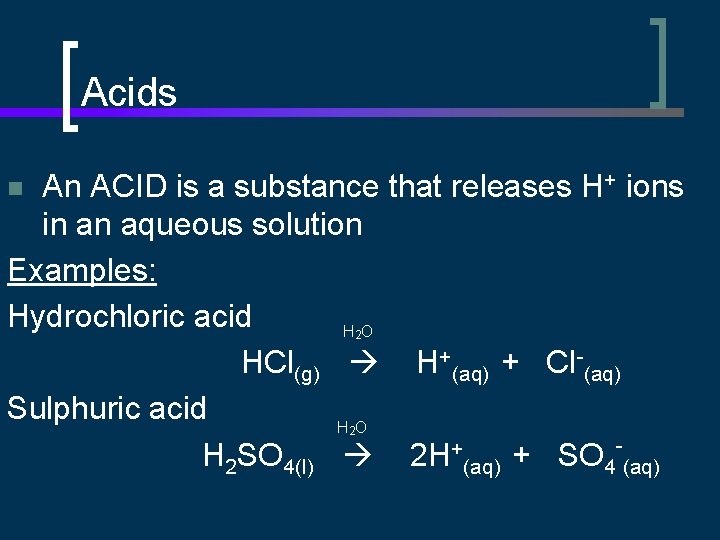
Acids An ACID is a substance that releases H+ ions in an aqueous solution Examples: Hydrochloric acid H O HCl(g) H+(aq) + Cl-(aq) Sulphuric acid H O H 2 SO 4(l) 2 H+(aq) + SO 4 -(aq) n 2 2

Bases n n n Taste bitter Feel slippery Change the color of indicators - Red litmus turns blue

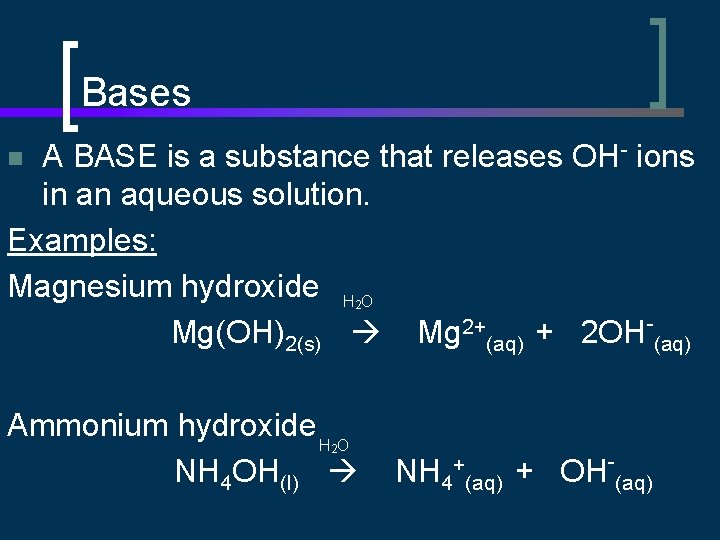
Bases A BASE is a substance that releases OH- ions in an aqueous solution. Examples: Magnesium hydroxide H O Mg(OH)2(s) Mg 2+(aq) + 2 OH-(aq) n 2 Ammonium hydroxide H O NH 4 OH(l) 2 NH 4+(aq) + OH-(aq)

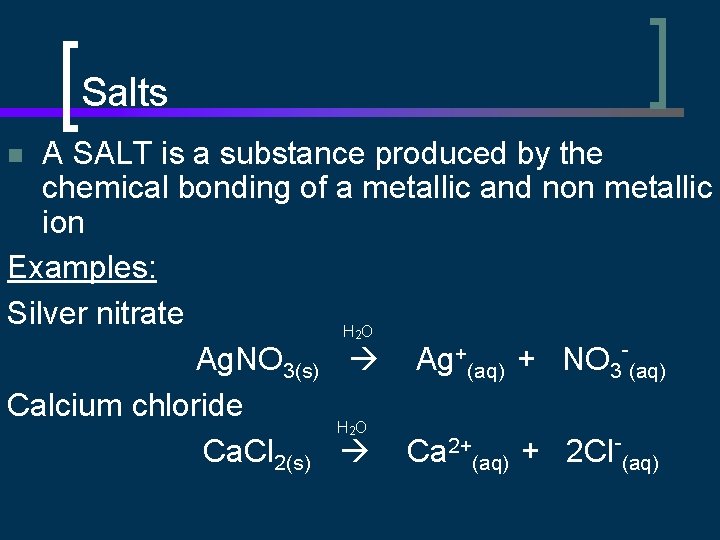
Salts A SALT is a substance produced by the chemical bonding of a metallic and non metallic ion Examples: Silver nitrate H O Ag. NO 3(s) Ag+(aq) + NO 3 -(aq) Calcium chloride H O Ca. Cl 2(s) Ca 2+(aq) + 2 Cl-(aq) n 2 2

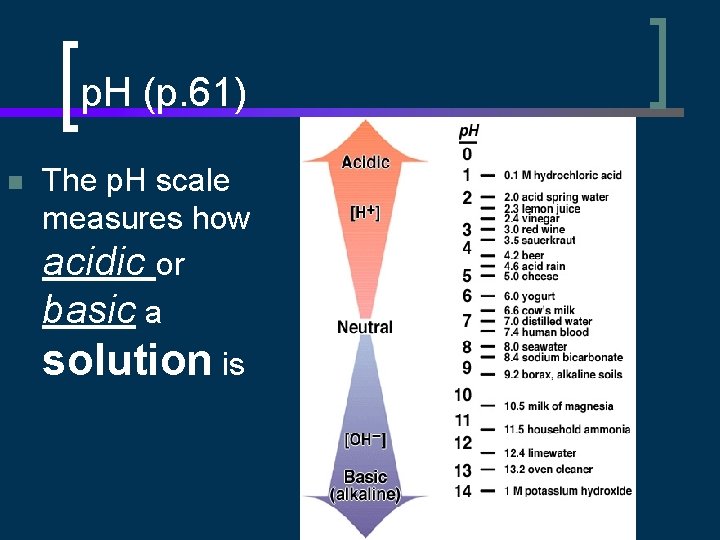
p. H (p. 61) n The p. H scale measures how acidic or basic a solution is

Identifying Acids and Bases n Acids: p. H < 7 n Lower p. H stronger acid n Bases: p. H > 7 n Higher p. H stronger base Neutral substances n p. H = 7 n

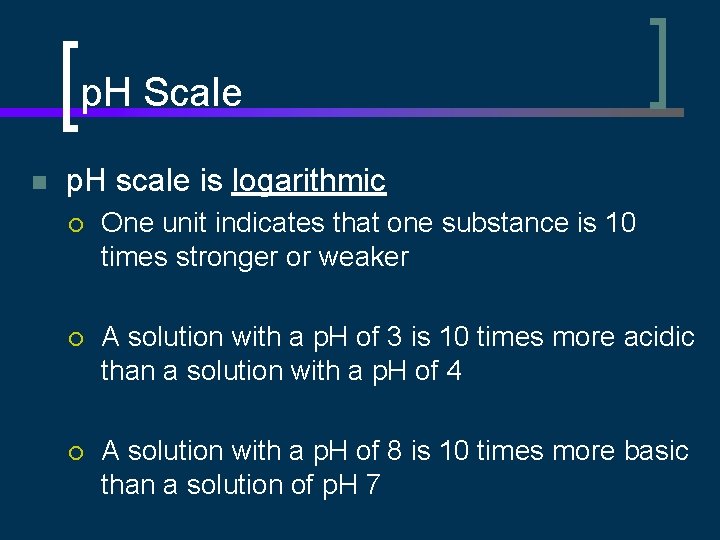
p. H Scale n p. H scale is logarithmic ¡ One unit indicates that one substance is 10 times stronger or weaker ¡ A solution with a p. H of 3 is 10 times more acidic than a solution with a p. H of 4 ¡ A solution with a p. H of 8 is 10 times more basic than a solution of p. H 7

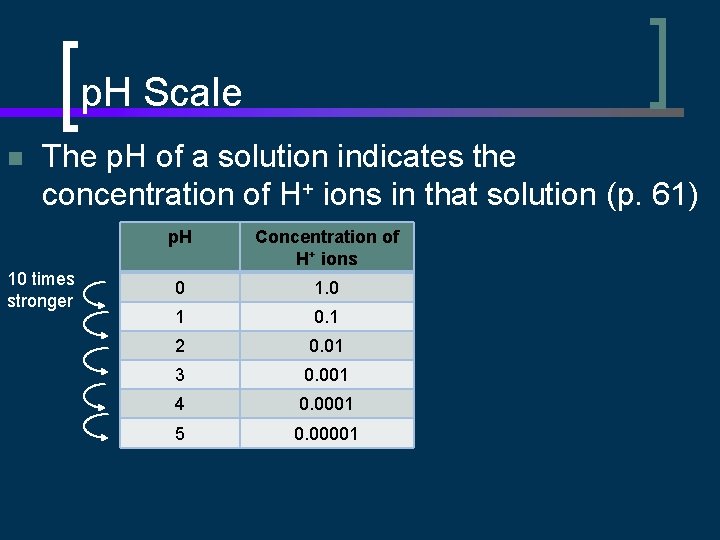
p. H Scale n The p. H of a solution indicates the concentration of H+ ions in that solution (p. 61) 10 times stronger p. H Concentration of H+ ions 0 1 0. 1 2 0. 01 3 0. 001 4 0. 0001 5 0. 00001

p. H Scale How many more times weaker is a solution with p. H 6 compared to a solution of p. H 3? 10 x 10 = 1000

Types of Chemical Reactions

Oxidation Reactions n Oxidation is a chemical change involving oxygen (or a substance with properties similar to oxygen) n Reactions with metals: metal + O 2(g) metal oxide

Oxidation Reactions Examples 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) Iron oxide is also known as rust 2 Cu(s) + O 2(g) 2 Cu. O(s)

Types Combustion of Chemical Reactions n Combustion is a form of oxidation that releases a large amount of energy ¡ Fuel + Oxygen Carbon dioxide + Water Examples: CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l)

Combustion Reactions n 3 conditions must be fulfilled for combustion ¡ Fuel (compounds with C and H) ¡ ¡ Oxygen Heat Oxygen (to reach the ignition temperature) Fuel Heat

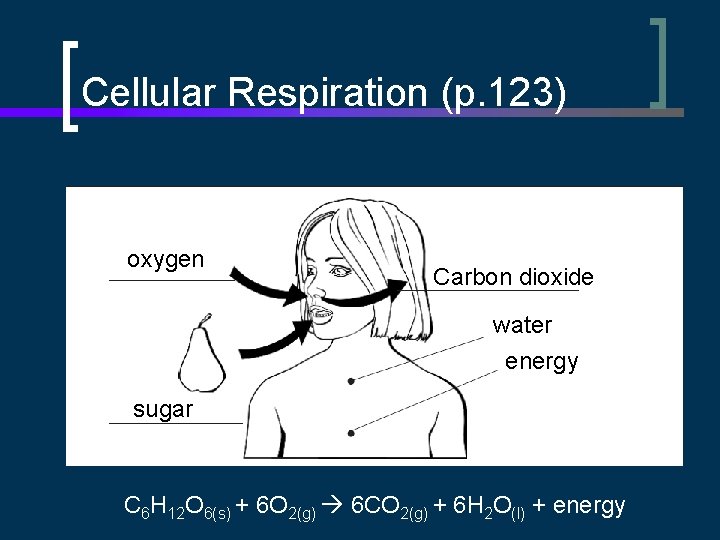
Cellular Respiration n A form of slow combustion C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l) + energy n n Occurs in cells of most living organisms Energy produced disperses to surrounding tissues

Cellular Respiration (p. 123) oxygen Carbon dioxide water energy sugar C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l) + energy

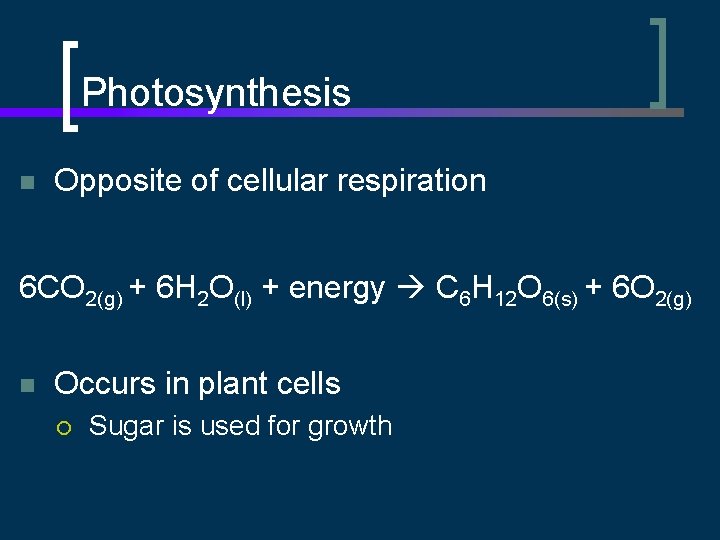
Photosynthesis n Opposite of cellular respiration 6 CO 2(g) + 6 H 2 O(l) + energy C 6 H 12 O 6(s) + 6 O 2(g) n Occurs in plant cells ¡ Sugar is used for growth

Photosynthesis (p. 124) oxygen Solar energy Carbon dioxide sugar water 6 CO 2(g) + 6 H 2 O(l) + energy C 6 H 12 O 6(s) + 6 O 2(g)

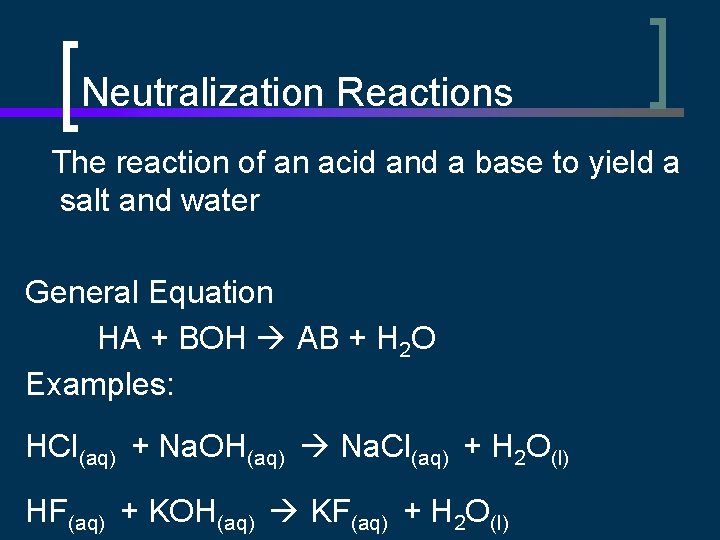
Neutralization Reactions The reaction of an acid and a base to yield a salt and water General Equation HA + BOH AB + H 2 O Examples: HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) HF(aq) + KOH(aq) KF(aq) + H 2 O(l)

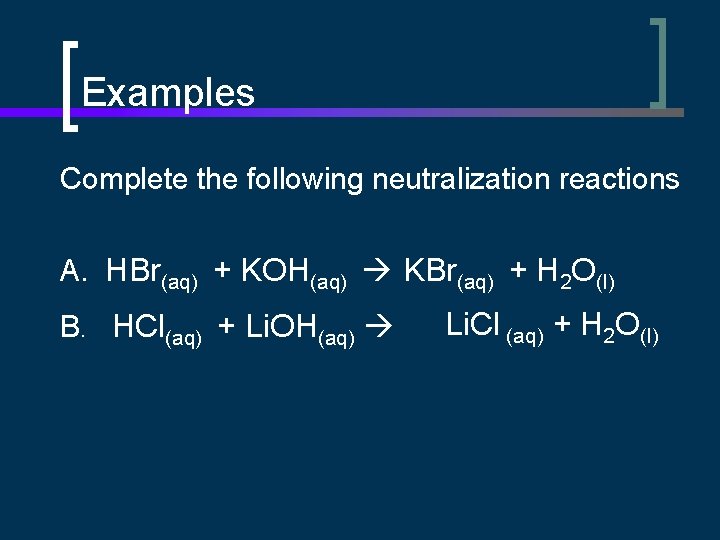
Examples Complete the following neutralization reactions A. HBr(aq) + KOH(aq) KBr(aq) + H 2 O(l) B. HCl(aq) + Li. OH(aq) Li. Cl (aq) + H 2 O(l)

Conservation of Mass n “Nothing is lost; nothing is created; everything is transformed” Antoine Laurent de Lavoisier The LAW OF CONSERVATION OF MASS states that the total mass of reactants is always equal to the total mass of products in a chemical reaction

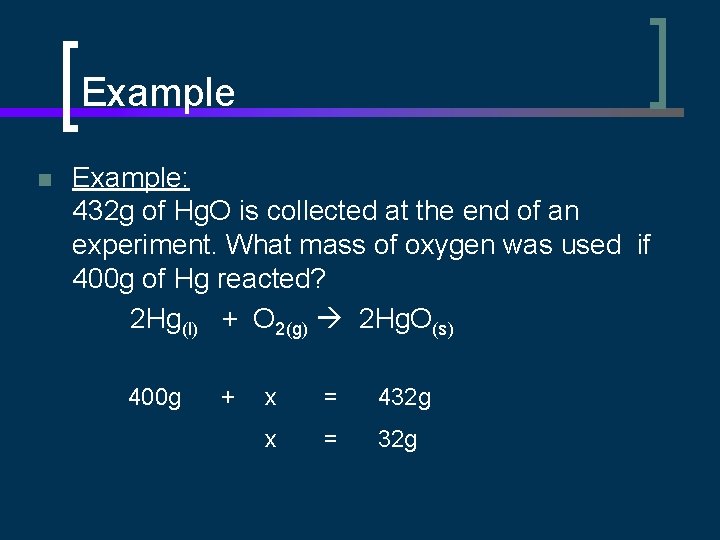
Example n Example: 432 g of Hg. O is collected at the end of an experiment. What mass of oxygen was used if 400 g of Hg reacted? 2 Hg(l) + O 2(g) 2 Hg. O(s) 400 g + x = 432 g x = 32 g

Balancing Chemical Reactions
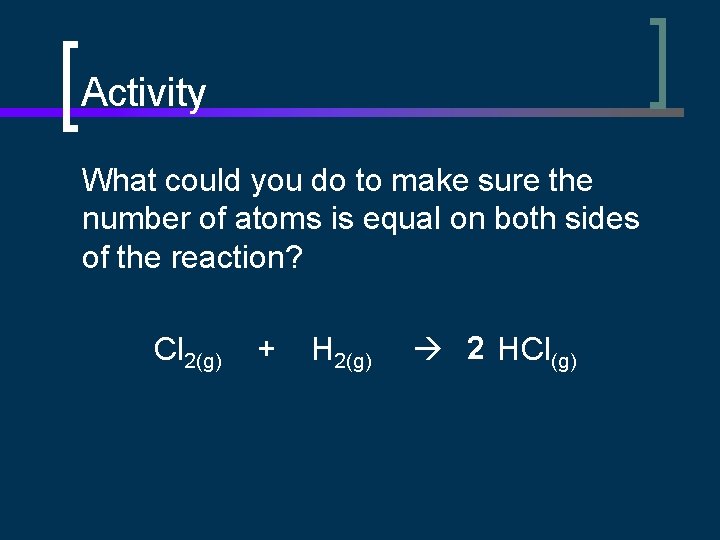
Activity What could you do to make sure the number of atoms is equal on both sides of the reaction? Cl 2(g) + H 2(g) 2 HCl(g)

Balancing Chemical Equations n Balancing ensures that the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side ¡ This ensures the conservation of mass

Rules for Balancing 1. 2. 3. Coefficients must be whole numbers in the smallest ratio Subscripts in chemical formulas must NEVER be changed New substances cannot be added or removed

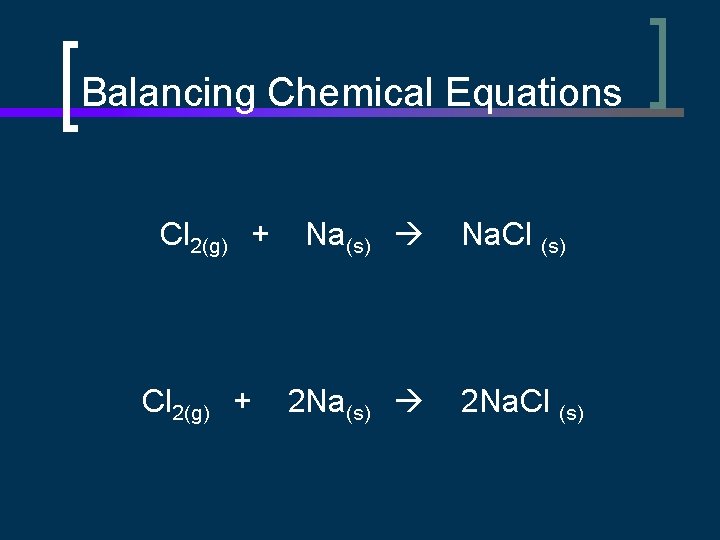
Balancing Chemical Equations Cl 2(g) + Na(s) Na. Cl (s) 2 Na(s) 2 Na. Cl (s)

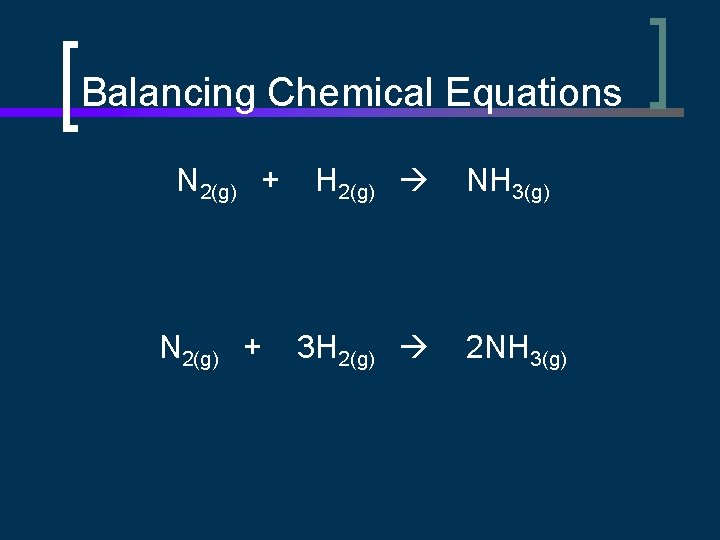
Balancing Chemical Equations N 2(g) + H 2(g) NH 3(g) 3 H 2(g) 2 NH 3(g)

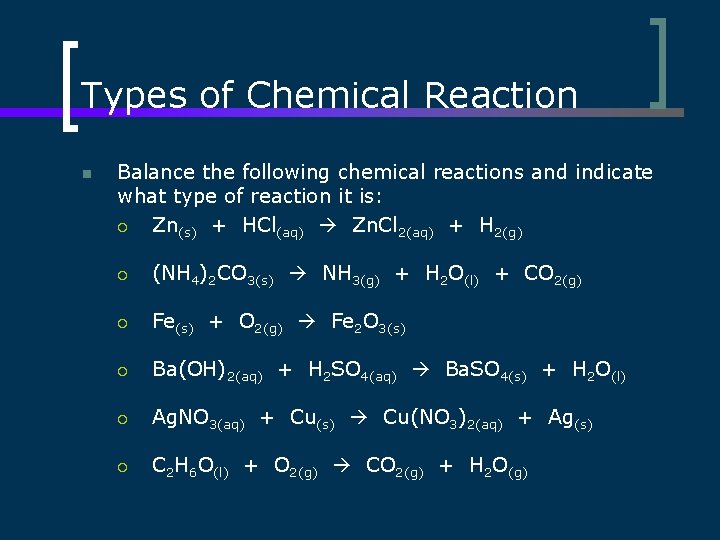
Types of Chemical Reaction n Balance the following chemical reactions and indicate what type of reaction it is: ¡ Zn(s) + HCl(aq) Zn. Cl 2(aq) + H 2(g) ¡ (NH 4)2 CO 3(s) NH 3(g) + H 2 O(l) + CO 2(g) ¡ Fe(s) + O 2(g) Fe 2 O 3(s) ¡ Ba(OH)2(aq) + H 2 SO 4(aq) Ba. SO 4(s) + H 2 O(l) ¡ Ag. NO 3(aq) + Cu(s) Cu(NO 3)2(aq) + Ag(s) ¡ C 2 H 6 O(l) + O 2(g) CO 2(g) + H 2 O(g)