The Lysosome and Lysosomal Storage Disorders LSD Part










- Slides: 45

The Lysosome and Lysosomal Storage Disorders (LSD) Part I: Historical, Physiological and Pathological Considerations Serge Melançon, MD January 2010

Synopsis (Part 1 A) • Historical notes and scientific partners • The endosomal-lysosomal system • The ‘synthetic’ pathway • The ‘endocytotic’ pathway • Retrograde transport

Synopsis (Part 1 B) • • • Cofactors for lysosomal enzymes Secretion-recapture pathway Plasma membranes and lipid rafts Molecular genetics Microglia Blood-brain barrier (BBB) Causes of lysosomal storage Residual enzymatic activity Effects of lysosomal storage

The discovery of lysosomes Why? In 1955, Belgian scientist Christian de Duve observed that the cells released an enzyme called acid phosphatase in much larger amounts when they were repeatedly frozen and thawed before centrifugation de Duve C (1975) Exploring cells with a centrifuge. Science 189, 186 -194

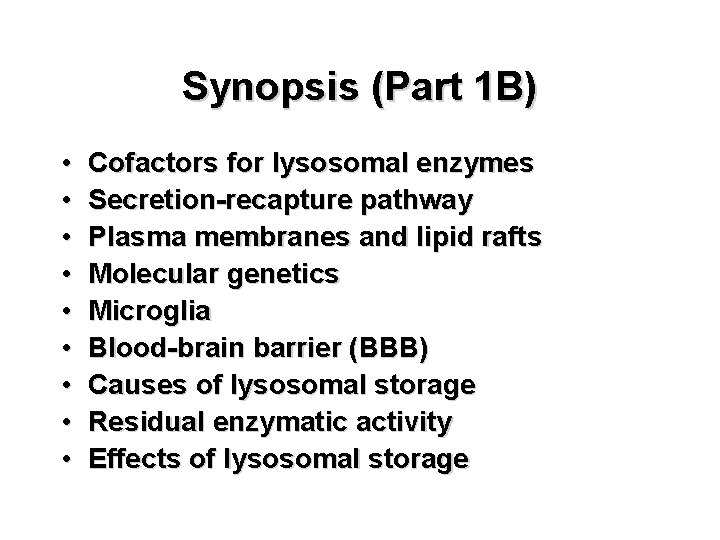
To explain this phenomenon, de Duve suggested that the digestive enzyme must have been encased in some sort of membrane-bound organelle within the cell.

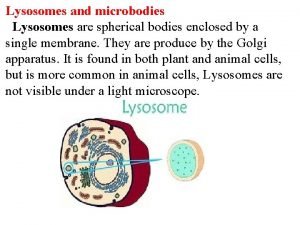
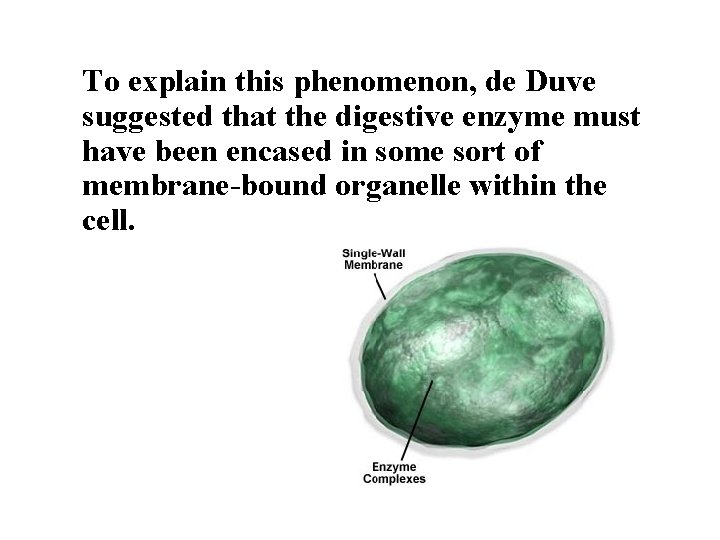
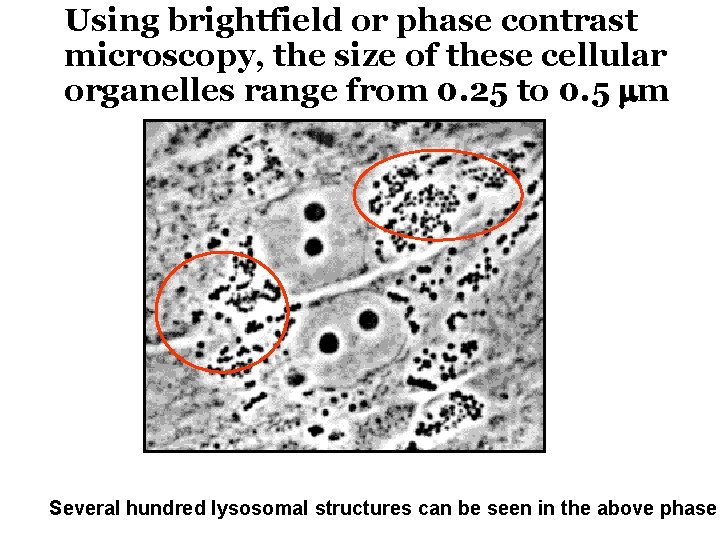
After estimating the probable size of the lysosomes, he was able to identify the organelle in images produced with an electron microscope Lysosomes are spherical organelles contained by a single layer membrane, though their size and shape vary to some extent.

Using brightfield or phase contrast microscopy, the size of these cellular organelles range from 0. 25 to 0. 5 mm Several hundred lysosomal structures can be seen in the above phase

• "Lysosome" was the name given because of these enzymes' ability to "lyse" the cell • Initially referred to as "suicide bags" since one of the functions of lysosomes is to rupture when the cell dies, • Rupturing releases hydrolytic enzymes that digest all parts of the cell, including proteins, DNA, RNA, carbohydrates, lipids and cellulose.

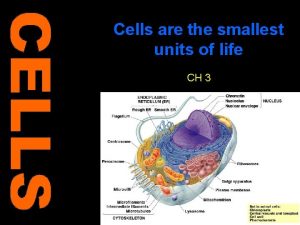
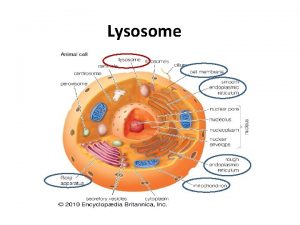
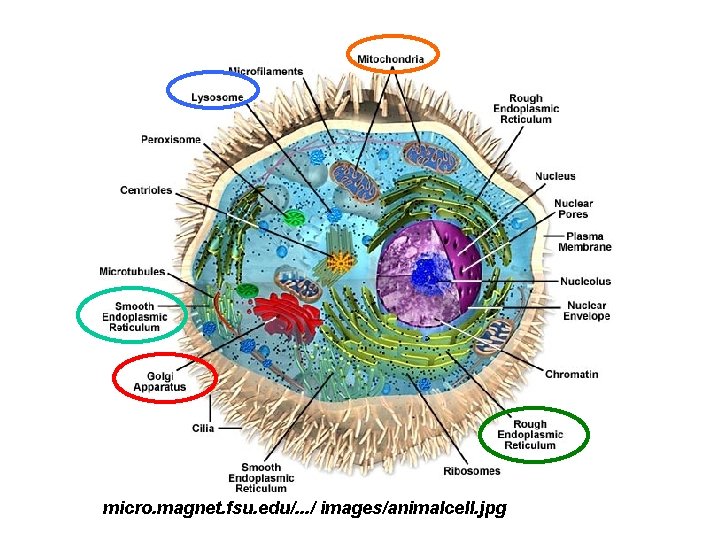
CELL GEOGRAPHY 101

micro. magnet. fsu. edu/. . . / images/animalcell. jpg

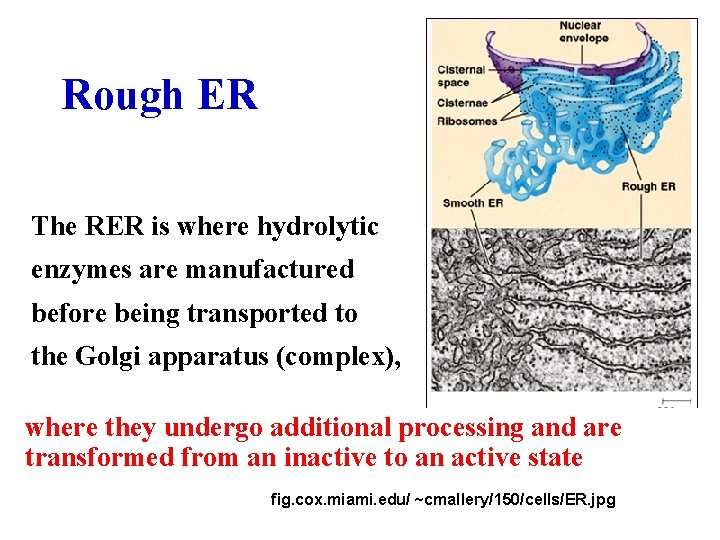
Rough ER The RER is where hydrolytic enzymes are manufactured before being transported to the Golgi apparatus (complex), where they undergo additional processing and are transformed from an inactive to an active state fig. cox. miami. edu/ ~cmallery/150/cells/ER. jpg

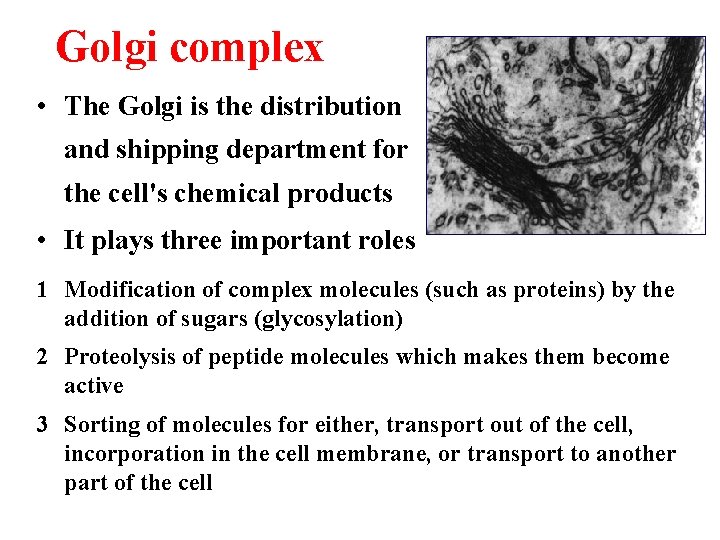
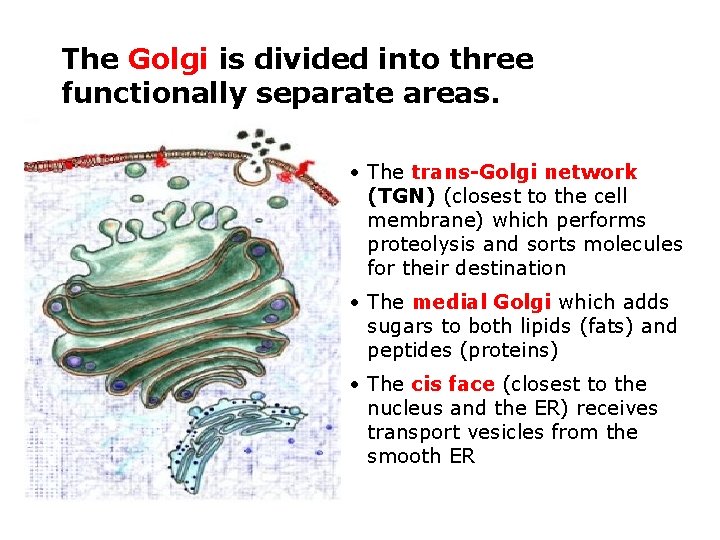
Golgi complex • The Golgi is the distribution and shipping department for the cell's chemical products • It plays three important roles 1 Modification of complex molecules (such as proteins) by the addition of sugars (glycosylation) 2 Proteolysis of peptide molecules which makes them become active 3 Sorting of molecules for either, transport out of the cell, incorporation in the cell membrane, or transport to another part of the cell

The Golgi is divided into three functionally separate areas. • The trans-Golgi network (TGN) (closest to the cell membrane) which performs proteolysis and sorts molecules for their destination • The medial Golgi which adds sugars to both lipids (fats) and peptides (proteins) • The cis face (closest to the nucleus and the ER) receives transport vesicles from the smooth ER

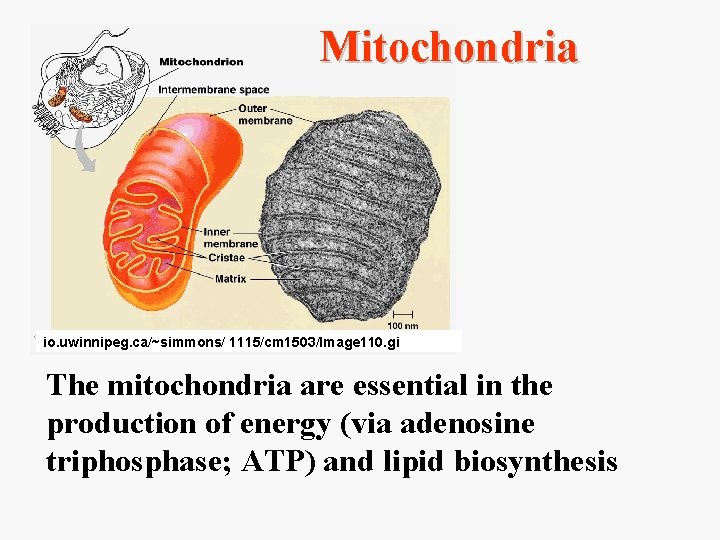
Mitochondria io. uwinnipeg. ca/~simmons/ 1115/cm 1503/Image 110. gi The mitochondria are essential in the production of energy (via adenosine triphosphase; ATP) and lipid biosynthesis

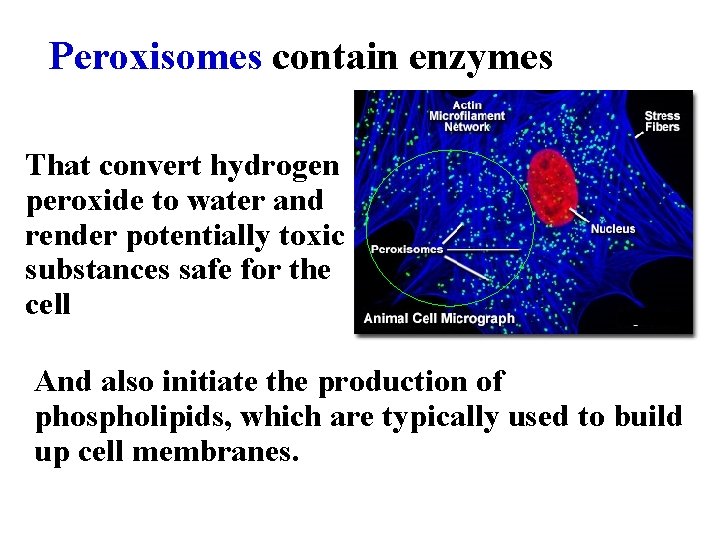
Peroxisomes contain enzymes That convert hydrogen peroxide to water and render potentially toxic substances safe for the cell And also initiate the production of phospholipids, which are typically used to build up cell membranes.

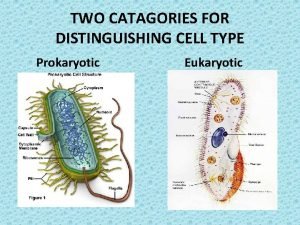
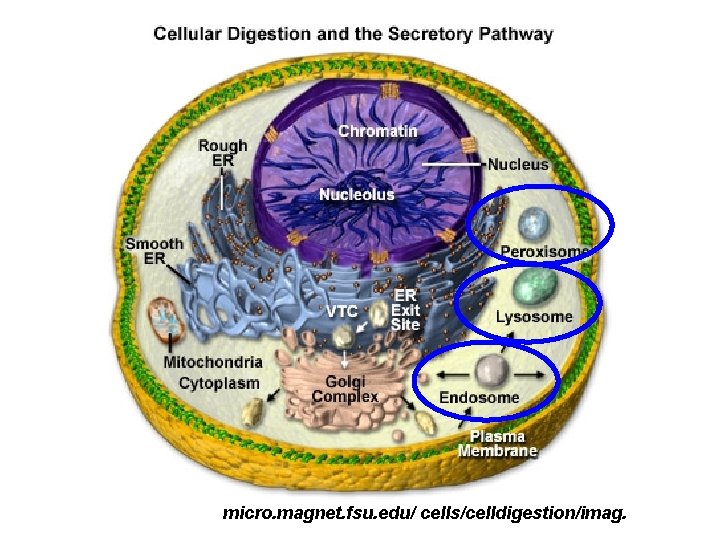
The endosomal–lysosomal system • The lysosome is one component of a series of unconnected (? ) intracellular organelles, intracellular organelles collectively known as the endosomal–lysosomal system or vacuolar apparatus (De Duve and Wattiaux, 1966) • The various components of the system were described over 30 years ago by Novikoff (1973)

micro. magnet. fsu. edu/ cells/celldigestion/imag.

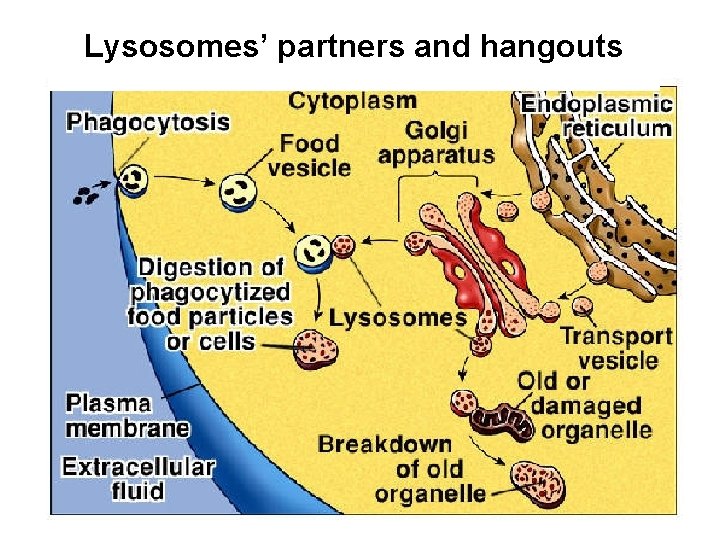
The endosomal–lysosomal system • The main components are – the early endosome, situated at the cell periphery, – the late endosome, which is perinuclear, and – the lysosome. • They form a chain that is responsible for – the trafficking and digestion of endocytosed molecules – and participate actively in sorting and recycling

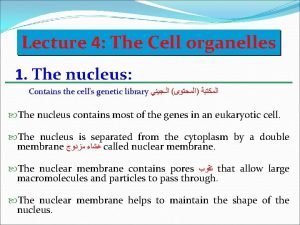
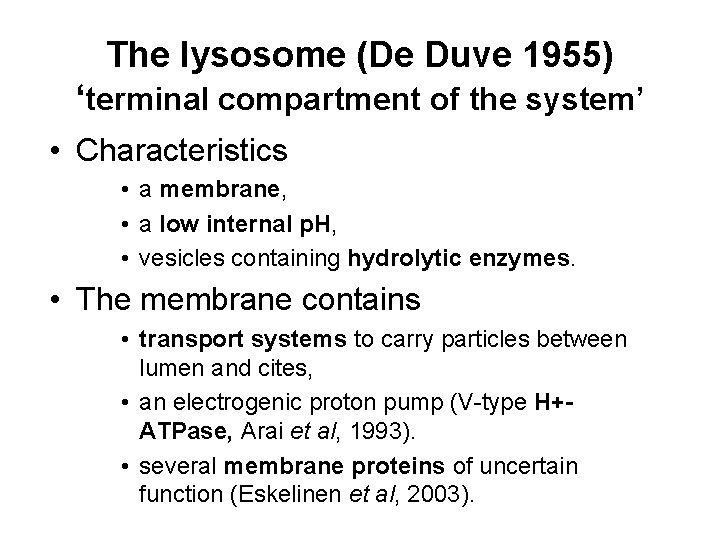
The lysosome (De Duve 1955) ‘terminal compartment of the system’ • Characteristics • a membrane, • a low internal p. H, • vesicles containing hydrolytic enzymes. • The membrane contains • transport systems to carry particles between lumen and cites, • an electrogenic proton pump (V-type H+ATPase, Arai et al, 1993). • several membrane proteins of uncertain function (Eskelinen et al, 2003).

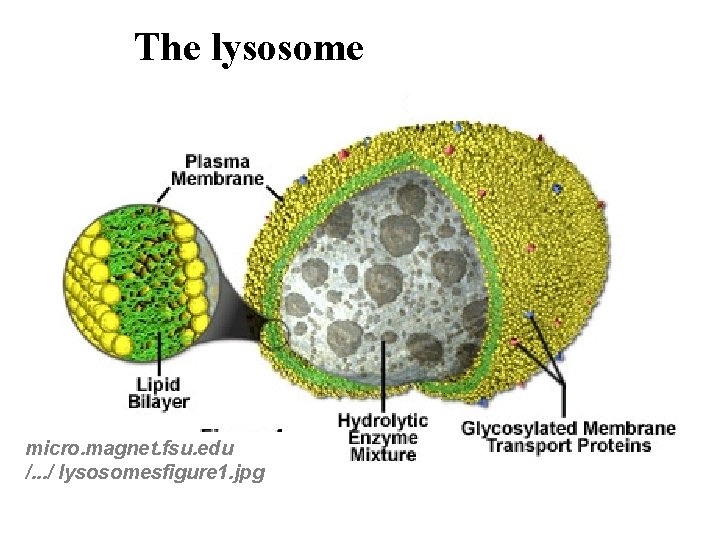
The lysosome micro. magnet. fsu. edu /. . . / lysosomesfigure 1. jpg

Role of the lysosome • • Substrate breakdown using endocytosis Secretion of its content after fusion with the plasma membrane (Luzio et al, 2000). • Phagocytosis of bacteria and cellular debris to form phagolysosomes. • Calcium-regulated exocytosis for membrane repair (Reddy et al, 2001)

Lysosomes’ partners and hangouts

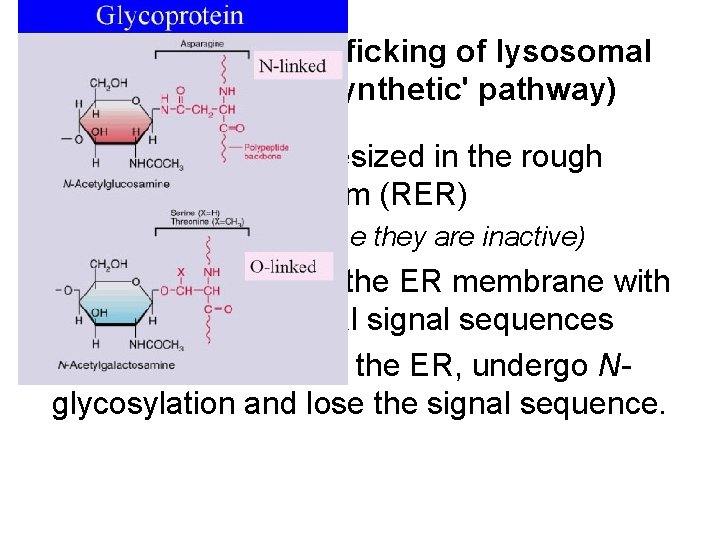
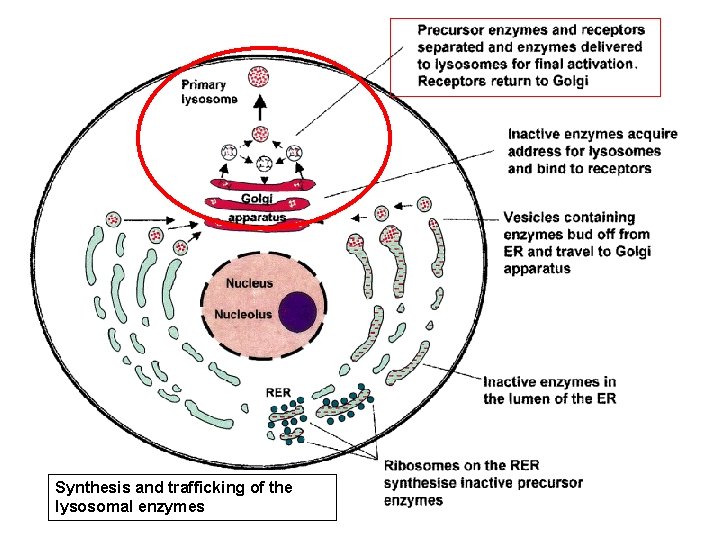
Synthesis and trafficking of lysosomal enzymes (the 'synthetic' pathway) • Glycoproteins synthesized in the rough endoplasmic reticulum (RER) (At this early stage they are inactive) • Translocate through the ER membrane with the help of N-terminal signal sequences • Once in the lumen of the ER, undergo Nglycosylation and lose the signal sequence.

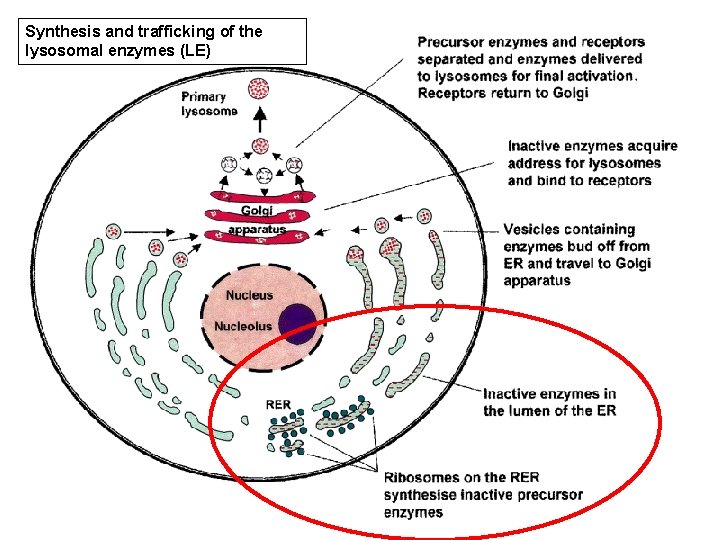
Synthesis and trafficking of the lysosomal enzymes (LE)

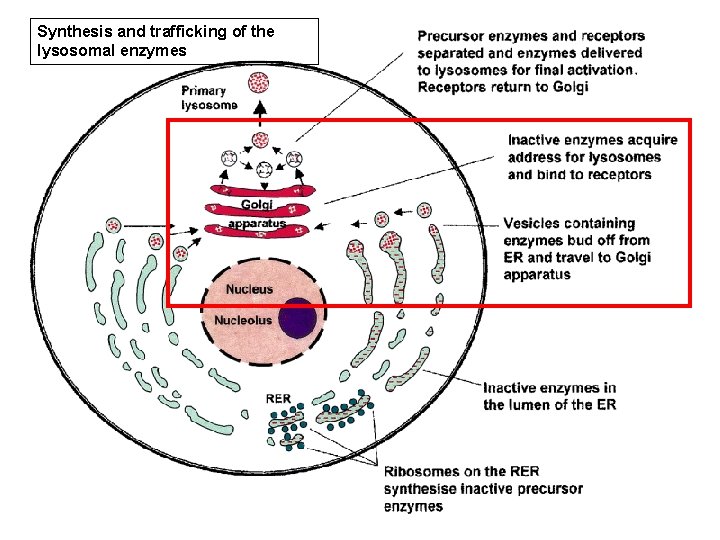
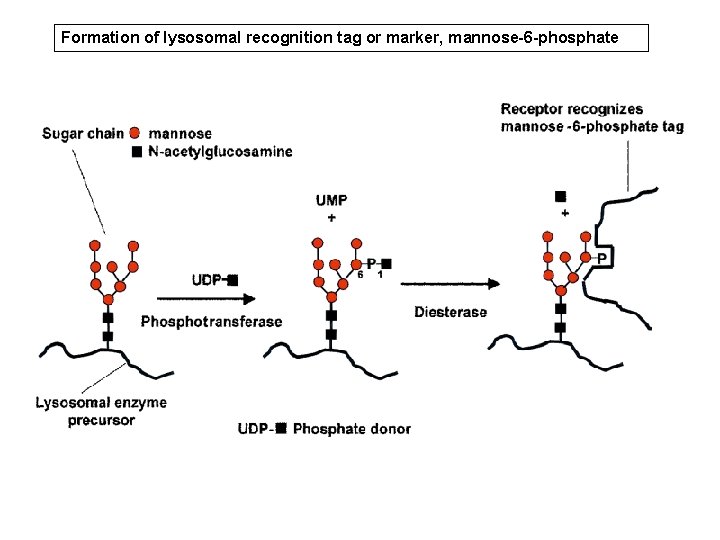
the 'synthetic' pathway • LE then move to the Golgi compartment to acquire mannose 6 -phosphate (M 6 - P) ligand (marker) • Process requires the sequential action of two enzymes, – a phosphotransferase (Reitman & Kornfeld, phosphotransferase 1981; Waheed et al, 1981) – a diesterase (Varki & Kornfeld, 1981; Waheed diesterase et al, 1981).

Synthesis and trafficking of the lysosomal enzymes

the 'synthetic' pathway • M 6 -P marker separates glycoproteins destined for the lysosome from secretory glycoproteins • Failure to acquire this marker results in mistargeting of lysosomal enzymes; they will not enter the lysosome and substrate breakdown will not occur.

Formation of lysosomal recognition tag or marker, mannose-6 -phosphate

the 'synthetic' pathway • In the mucolipidoses II (I-cell disease) and III (pseudo -Hurler polydystrophy, mistargetting is precisely what happens • These patients lack the first enzyme, i. e. the phosphotransferase • Since all enzymes requiring the M 6 -P marker fail to enter the lysosome, they end up outside the cell • Consequently, these patients have very high plasma levels of all lysosomal enzymes • This clinical finding led to the discovery of the M 6 -P ligand its receptor (Hickman & Neufeld, 1972).

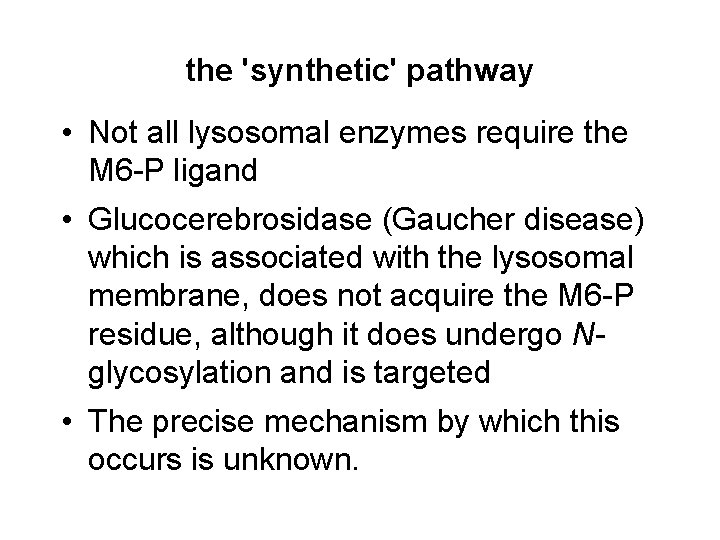
the 'synthetic' pathway • Not all lysosomal enzymes require the M 6 -P ligand • Glucocerebrosidase (Gaucher disease) which is associated with the lysosomal membrane, does not acquire the M 6 -P residue, although it does undergo Nglycosylation and is targeted • The precise mechanism by which this occurs is unknown.

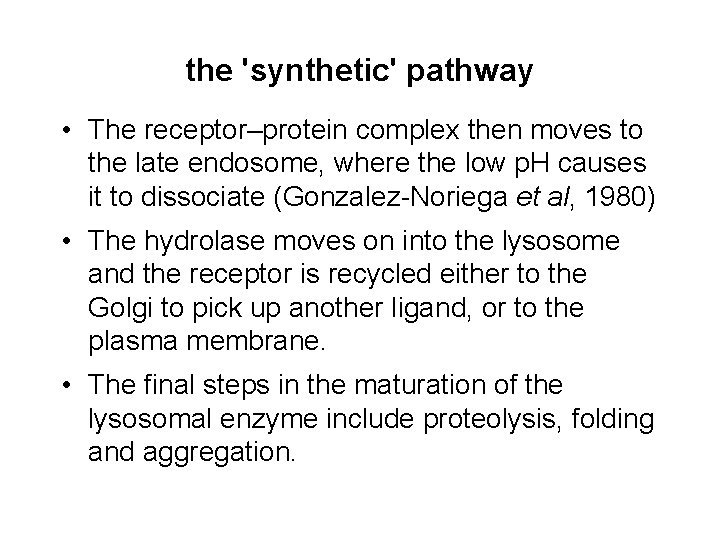
the 'synthetic' pathway • The receptor–protein complex then moves to the late endosome, where the low p. H causes it to dissociate (Gonzalez-Noriega et al, 1980) • The hydrolase moves on into the lysosome and the receptor is recycled either to the Golgi to pick up another ligand, or to the plasma membrane. • The final steps in the maturation of the lysosomal enzyme include proteolysis, folding and aggregation.

Synthesis and trafficking of the lysosomal enzymes

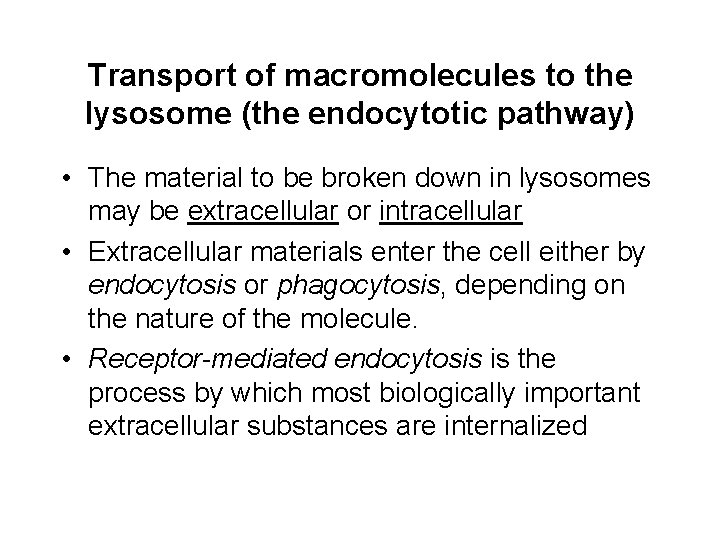
Transport of macromolecules to the lysosome (the endocytotic pathway) • The material to be broken down in lysosomes may be extracellular or intracellular • Extracellular materials enter the cell either by endocytosis or phagocytosis, depending on the nature of the molecule. • Receptor-mediated endocytosis is the process by which most biologically important extracellular substances are internalized

The endocytotic pathway

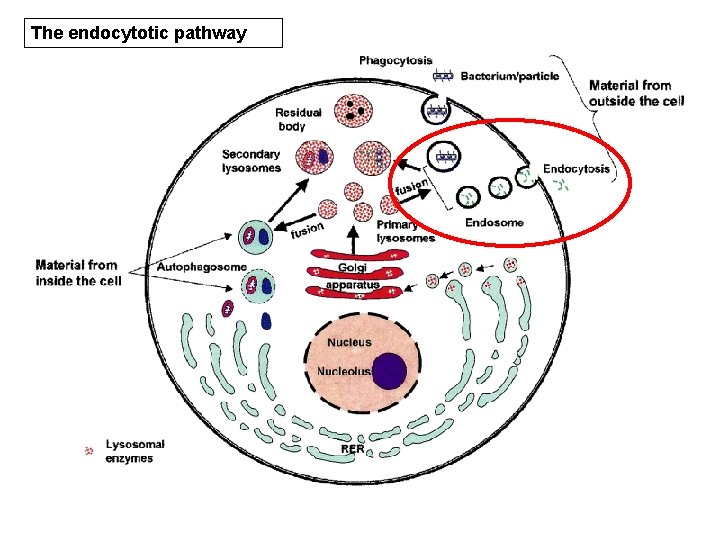
the ‘endocytotic’ pathway • This occurs by binding to specific cell surface receptors (Goldstein et al, 1985) • Ligands are first delivered to early endosomes, and then transported to late endosomes, probably by multivesicular bodies • They are then delivered to the lysosomes.

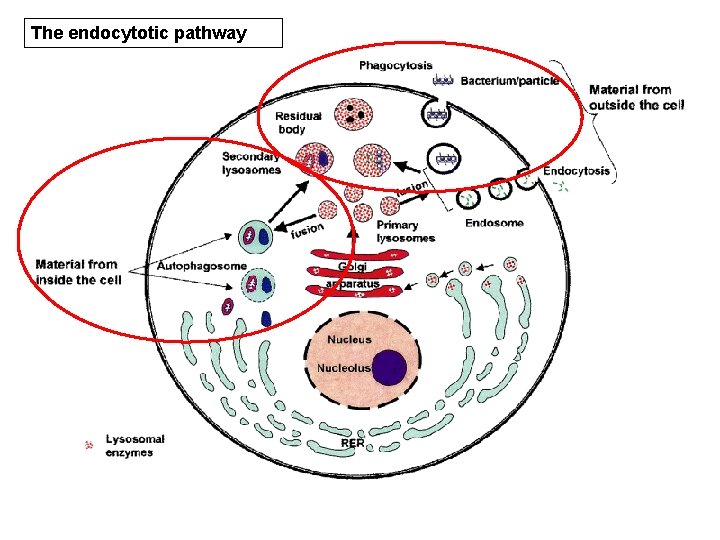
the ‘endocytotic’ pathway • Phagocytosis is the route of entry into the cell for microorganisms and cellular debris • Such particles are incorporated into phagosomes, which fuse with primary lysosomes to form secondary lysosomes • Finally, intracellular materials undergo autophagy

The endocytotic pathway

the ‘endocytotic’ pathway • Although a small amount of hydrolysis takes place in endosomes, the bulk of it takes place in the lysosome • This is because it is only in the acid milieu of the lysosome that hydrolases are active • The low p. H of the lysosome is maintained by the vacuolar proton pump

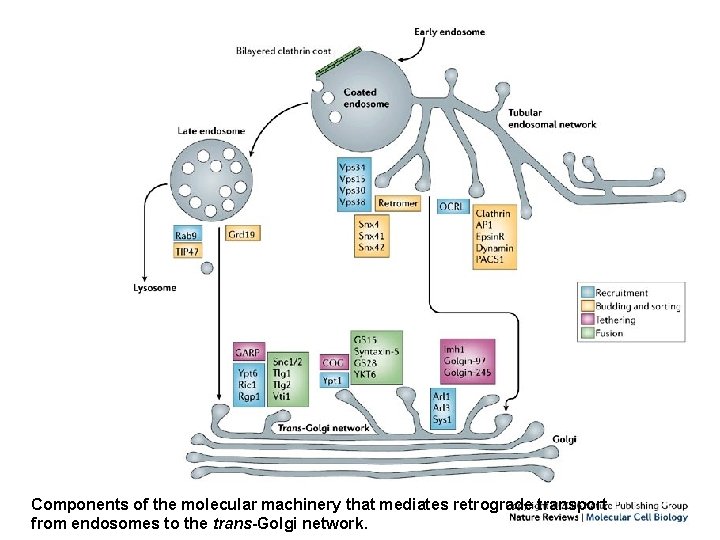
Retrograde transport from endosomes to the trans-Golgi network • Some intracellular transmembrane proteins, such as acid-hydrolase receptors, processing peptidases and SNAREs*, undergo retrograde transport from endosomes to the trans-Golgi network (TGN) as part of their normal trafficking • This retrograde-transport pathway is exploited by a subset of bacterial and plant protein toxins to enable them to reach the endoplasmic reticulum and eventually the cytosol. *Soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein receptors

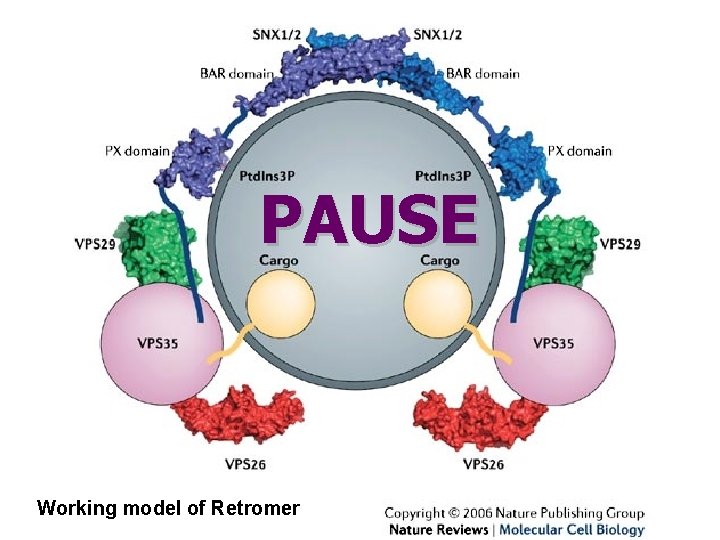
Retrograde transport • Recent studies have begun to unravel the molecular machinery that is involved in this retrograde transport. • Acid-hydrolase receptors such as S. cerevisiae vacuolar sorting-10 protein (Vps 10) and the mammalian mannose 6 -phosphate receptors (MPRs) are selected for retrograde transport by a five-subunit complex named 'retromer' • In mammalian cells, this complex is mainly associated with tubules that emanate from vacuolar, early–late endosomal intermediates.

Retrograde transport • Although MPRs seem to be capable of being transported to the TGN from both early and late endosomal compartments, protein toxins undergo retrograde transport exclusively from early endosomes. • Not surprisingly then, protein toxins and MPRs share some components of the retrogradetransport machinery that is associated with early endosomal compartments

Retrograde transport • The location of molecular devices that are involved in the retrograde transport of MPRs and protein toxins highlights the existence of an extensive 'tubular endosomal network' (TEN) • This TEN sorts and recycles cargoes to various destinations, including different domains of the plasma membrane, the limiting membrane of lysosomes and lysosome-related organelles, and specialized storage vesicles, in addition to the TGN

Components of the molecular machinery that mediates retrograde transport from endosomes to the trans-Golgi network.

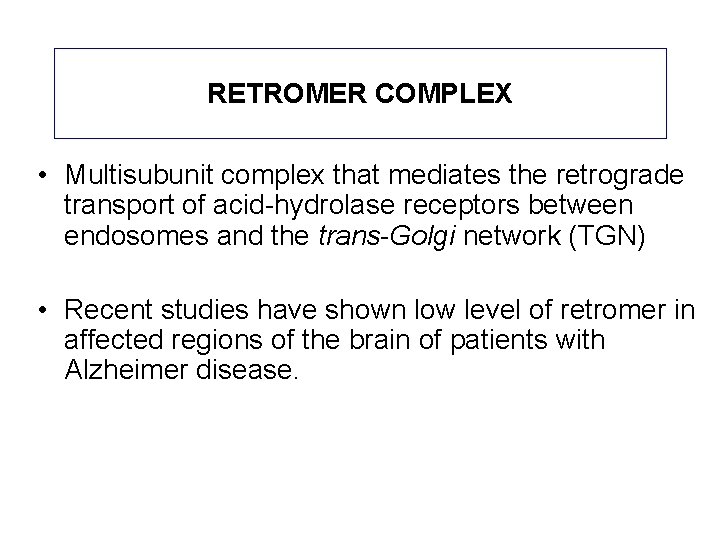
RETROMER COMPLEX • Multisubunit complex that mediates the retrograde transport of acid-hydrolase receptors between endosomes and the trans-Golgi network (TGN) • Recent studies have shown low level of retromer in affected regions of the brain of patients with Alzheimer disease.

PAUSE Working model of Retromer
 Primary lysosome vs secondary lysosome
Primary lysosome vs secondary lysosome Lysosomal disorders
Lysosomal disorders Lysosomal hydrolase
Lysosomal hydrolase Lysosomal enzymes
Lysosomal enzymes Sfingolipidosi
Sfingolipidosi Lysosomal hydrolase
Lysosomal hydrolase What is the function of lysosomes
What is the function of lysosomes Biology
Biology Lysosome location
Lysosome location Description
Description Lysosome facts
Lysosome facts Image of plant cell
Image of plant cell Lysosome simple definition
Lysosome simple definition Origin of lysosomes
Origin of lysosomes Struktur dan fungsi badan golgi
Struktur dan fungsi badan golgi Lysosome fungsi
Lysosome fungsi What organelle looks like a stack of pancakes
What organelle looks like a stack of pancakes Lysosome poem
Lysosome poem Sta je lsd
Sta je lsd Gesprekstechnieken lsd
Gesprekstechnieken lsd Lsd construction
Lsd construction Gesprekstechnieken lsd
Gesprekstechnieken lsd Wedervraag voorbeeld
Wedervraag voorbeeld Cone type lsd
Cone type lsd Lsd gesprekstechnieken
Lsd gesprekstechnieken Kanabis
Kanabis Synteticke drogy
Synteticke drogy Legalne lsd
Legalne lsd Lsd healthline
Lsd healthline Jens reinhardt
Jens reinhardt Transferring of data from auxiliary storage to main storage
Transferring of data from auxiliary storage to main storage Storage devices of computer
Storage devices of computer Secondary storage provides temporary or volatile storage
Secondary storage provides temporary or volatile storage Unified storage vs traditional storage
Unified storage vs traditional storage Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ