The Light Equations Calculations Bromfield Honors Chemistry Objectives

The Light Equations: Calculations Bromfield Honors Chemistry

Objectives Review Video the light equations Solve sample problems

The key equations c = ln

The key equations c = ln E = hn Memorize these!
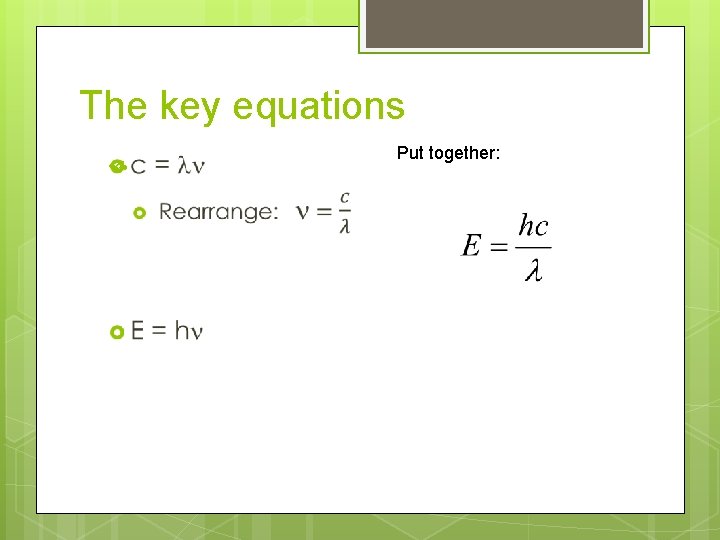
The key equations Put together:

The constants Speed of light in a vacuum c = 3. 00 x 108 m/s

The constants Speed of light in a vacuum c = 3. 00 x 108 m/s Planck’s constant h = 6. 626 x 10 -34 J s

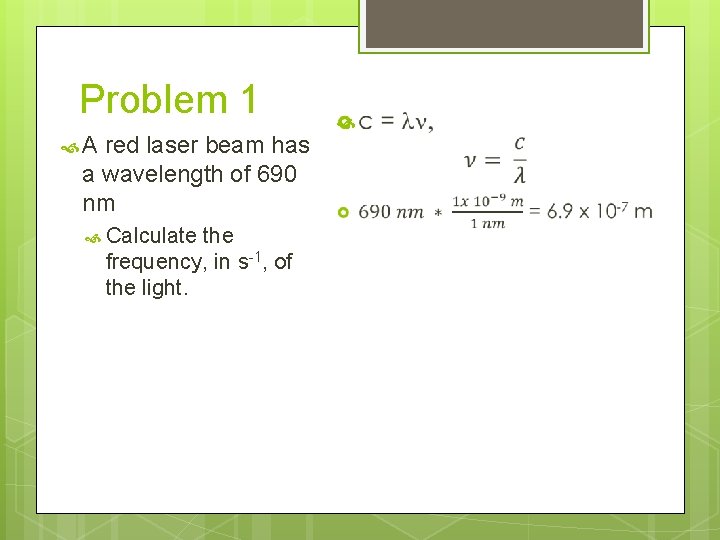
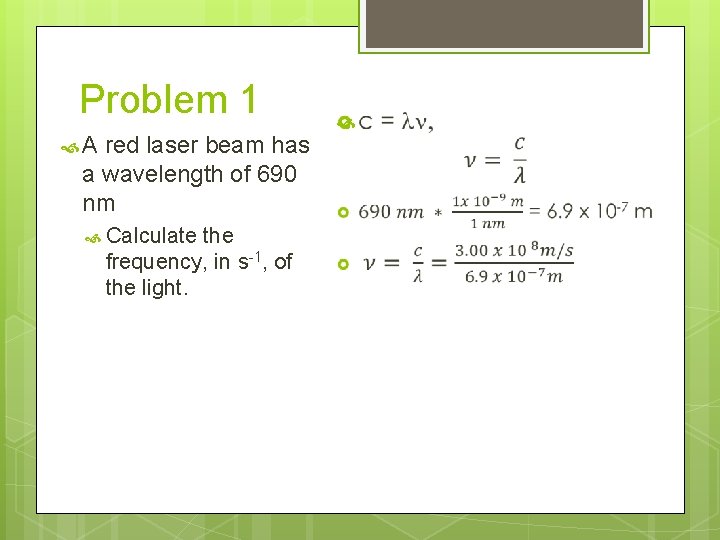
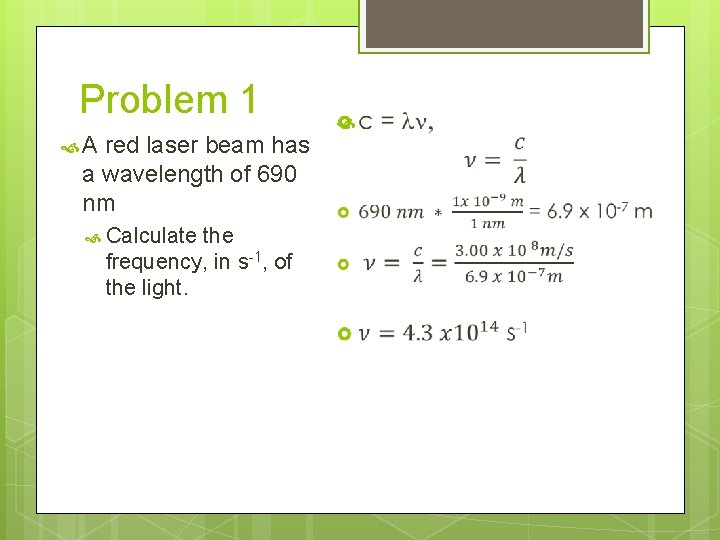
Problem 1 A red laser beam has a wavelength of 690 nm Calculate the frequency, in s-1, of the light.

Problem 1 A red laser beam has a wavelength of 690 nm Calculate the frequency, in s-1, of the light. c = ln

Problem 1 A red laser beam has a wavelength of 690 nm Calculate the frequency, in s-1, of the light.
Problem 1 A red laser beam has a wavelength of 690 nm Calculate the frequency, in s-1, of the light.
Problem 1 A red laser beam has a wavelength of 690 nm Calculate the frequency, in s-1, of the light.
Problem 1 A red laser beam has a wavelength of 690 nm Calculate the frequency, in s-1, of the light.

Problem 2 A red laser beam has a wavelength of 690 nm Calculate this light. the energy, in joules of one photon of

Problem 2 A red laser beam has a wavelength of 690 nm Calculate the energy, in joules of one photon of this light. E = hn, c = ln

Problem 2 A red laser beam has a wavelength of 690 nm Calculate the energy, in joules of one photon of this light.

Problem 2 A red laser beam has a wavelength of 690 nm Calculate the energy, in joules of one photon of this light.
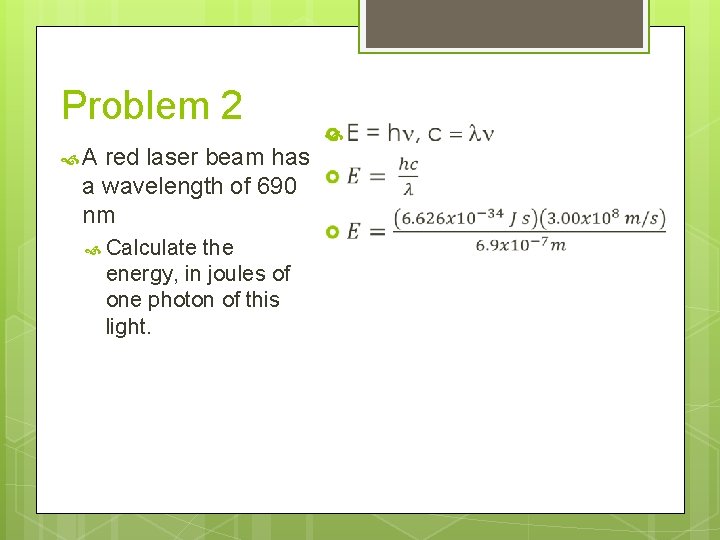
Problem 2 A red laser beam has a wavelength of 690 nm Calculate the energy, in joules of one photon of this light.
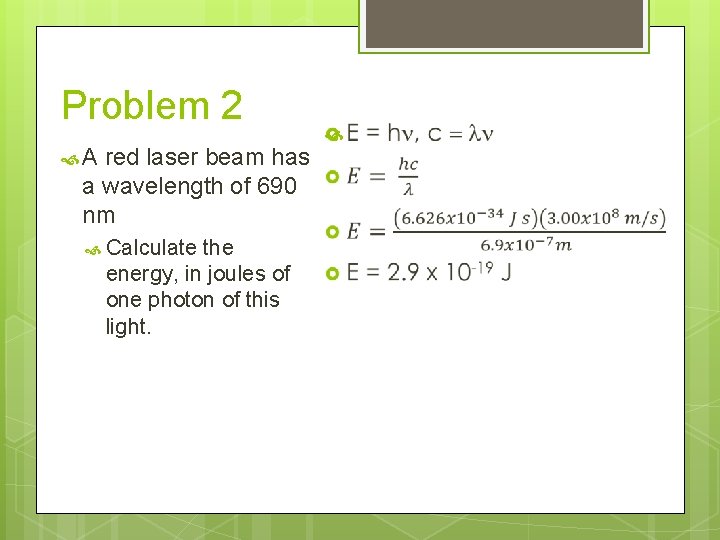
Problem 2 A red laser beam has a wavelength of 690 nm Calculate the energy, in joules of one photon of this light.
- Slides: 19