the light bulb is a reminder you need

the light bulb is a reminder you need to do something on your notes page


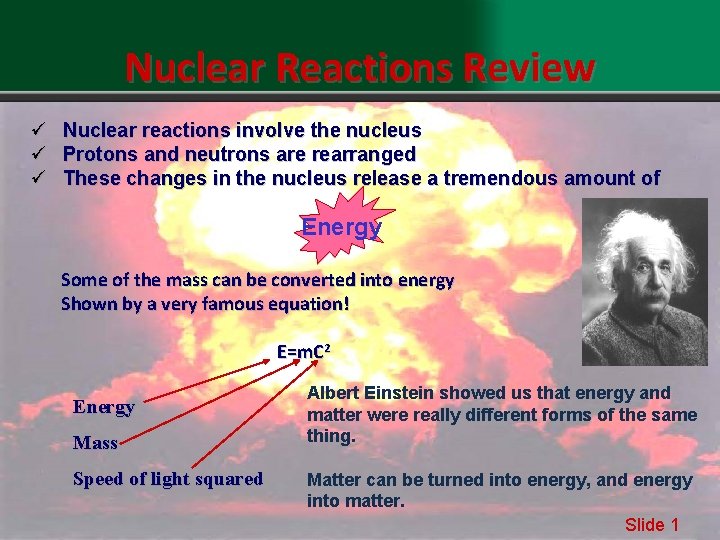
Nuclear Reactions Review ü Nuclear reactions involve the nucleus ü Protons and neutrons are rearranged ü These changes in the nucleus release a tremendous amount of Nuclear Reactions Energy Some of the mass can be converted into energy Shown by a very famous equation! E=m. C 2 Energy Mass Speed of light squared Albert Einstein showed us that energy and matter were really different forms of the same thing. Matter can be turned into energy, and energy into matter. Slide 1

E= m. C 2 Consider this: A single hydrogen atom (1 proton) has a mass of E = m. C 2 672 kg (not big) 0. 000 000 001 But everyday amounts of matter are made of LOTS of atoms 1 kg water = 111 g of hydrogen 111 g of Hydrogen = 10, 000, 000 Joules (energy) 1 Joule is not a lot of energy (about the same energy released as dropping a textbook to the floor) But 30 g of hydrogen has the equivalent energy as burning hundreds of thousands of gallons of gasoline Slide 2

Nuclear Reactions There are many kinds of nuclear reactions. 1. 2. 3. 4. Radioactive decay – alpha, beta, gamma Induced nuclear reactions – create unstable isotopes Fission reactions – a nucleus is split Fusion reactions – 2 or more nuclei join together Slide 3

1. Radioactive Decay The 3 MAIN types of radioactive decay a) Alpha decay b) Beta decay c) Gamma rays Slide 4
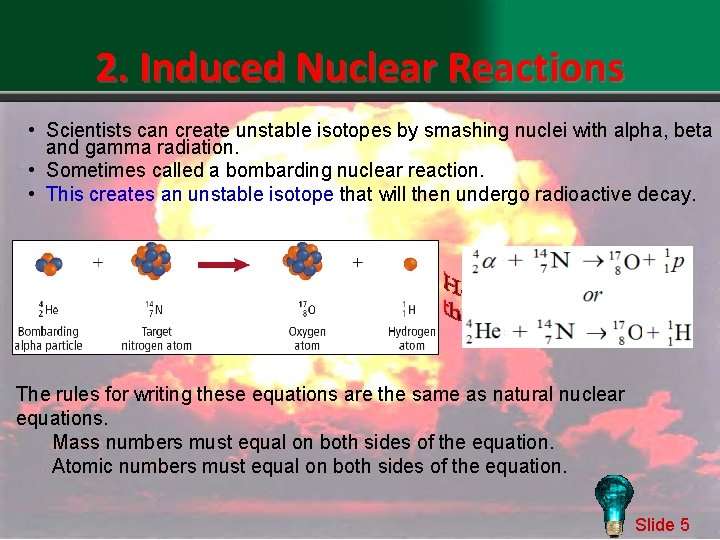
2. Induced Nuclear Reactions • Scientists can create unstable isotopes by smashing nuclei with alpha, beta and gamma radiation. • Sometimes called a bombarding nuclear reaction. • This creates an unstable isotope that will then undergo radioactive decay. The rules for writing these equations are the same as natural nuclear equations. Mass numbers must equal on both sides of the equation. Atomic numbers must equal on both sides of the equation. Slide 5

2. Induced Nuclear Reactions All of the known elements whose atomic number is greater than 92 were created from bombardment reactions Slide 6
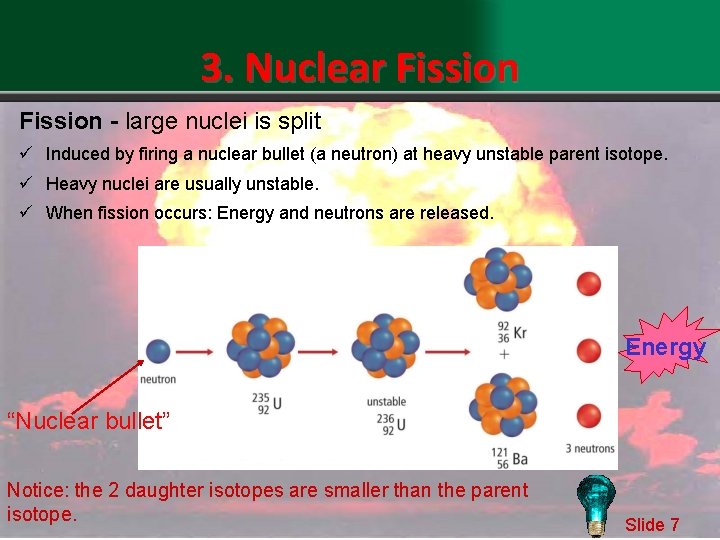
3. Nuclear Fission - large nuclei is split ü Induced by firing a nuclear bullet (a neutron) at heavy unstable parent isotope. ü Heavy nuclei are usually unstable. ü When fission occurs: Energy and neutrons are released. Energy “Nuclear bullet” Notice: the 2 daughter isotopes are smaller than the parent isotope. Slide 7
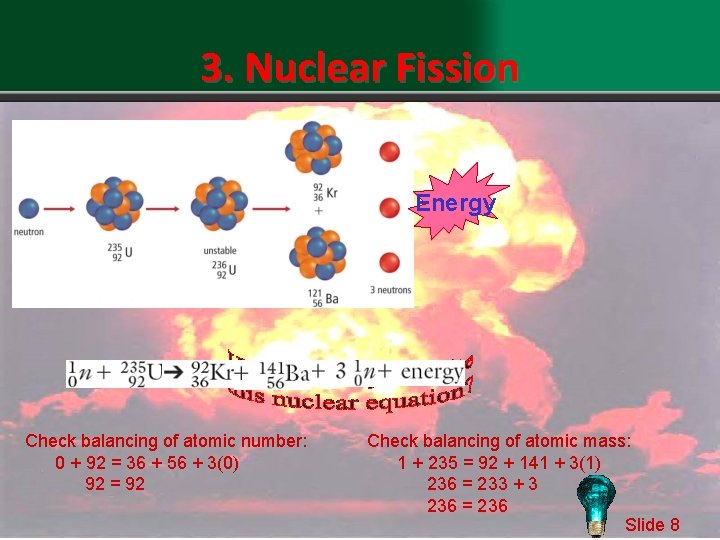
3. Nuclear Fission Energy Check balancing of atomic number: 0 + 92 = 36 + 56 + 3(0) 92 = 92 Check balancing of atomic mass: 1 + 235 = 92 + 141 + 3(1) 236 = 233 + 3 236 = 236 Slide 8
3. Nuclear Fission Slide 9

Nuclear Fission of Uranium - 235 The induced nuclear fission of uranium-235 provides the energy for MOST nuclear power plants and nuclear bombs. Slide 10
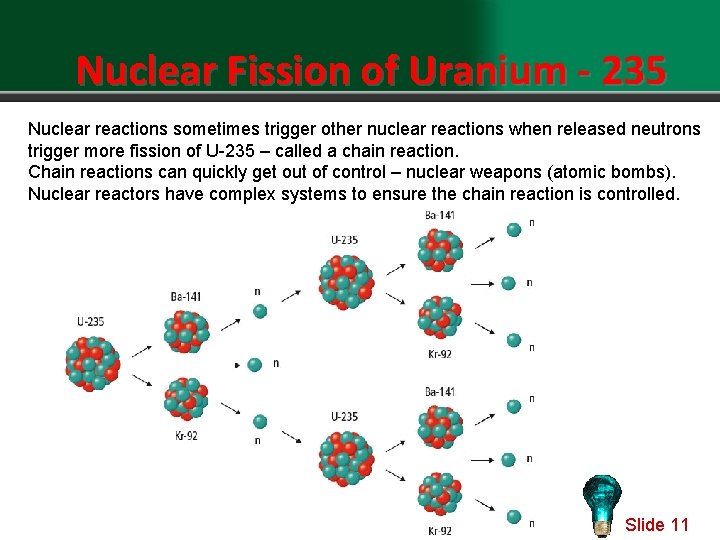
Nuclear Fission of Uranium - 235 Nuclear reactions sometimes trigger other nuclear reactions when released neutrons trigger more fission of U-235 – called a chain reaction. Chain reactions can quickly get out of control – nuclear weapons (atomic bombs). Nuclear reactors have complex systems to ensure the chain reaction is controlled. Slide 11

Nuclear Fission of Uranium - 235 Slide 12
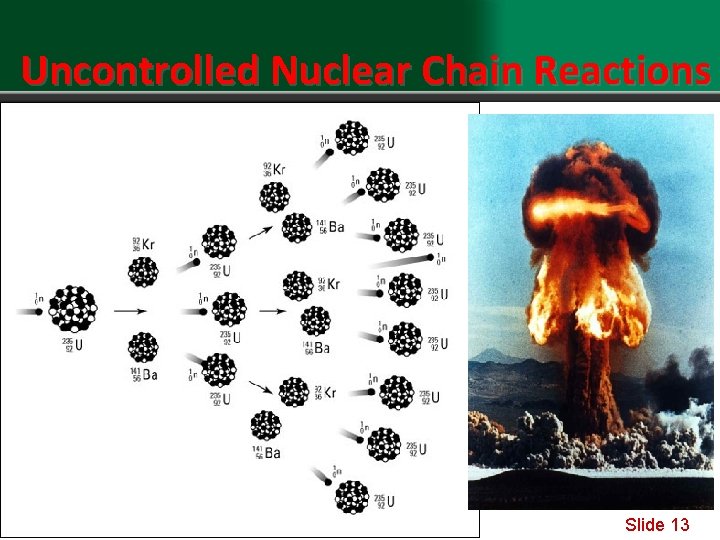
Uncontrolled Nuclear Chain Reactions Slide 13
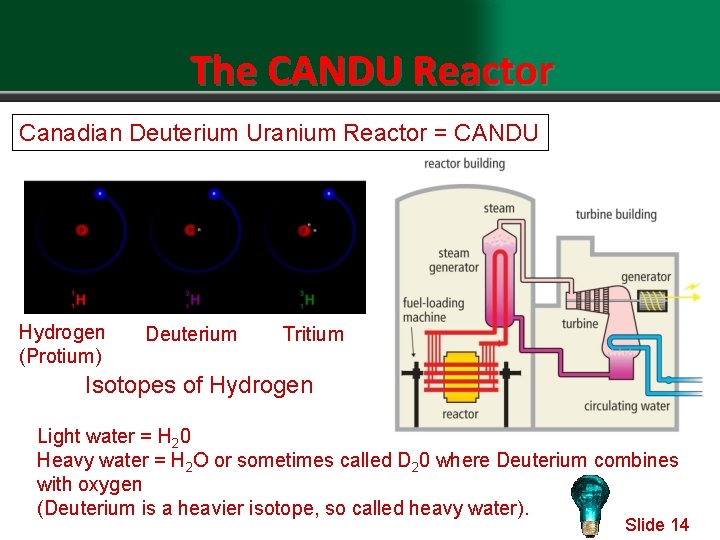
The CANDU Reactor Canadian Deuterium Uranium Reactor = CANDU Hydrogen (Protium) Deuterium Tritium Isotopes of Hydrogen Light water = H 20 Heavy water = H 2 O or sometimes called D 20 where Deuterium combines with oxygen (Deuterium is a heavier isotope, so called heavy water). Slide 14
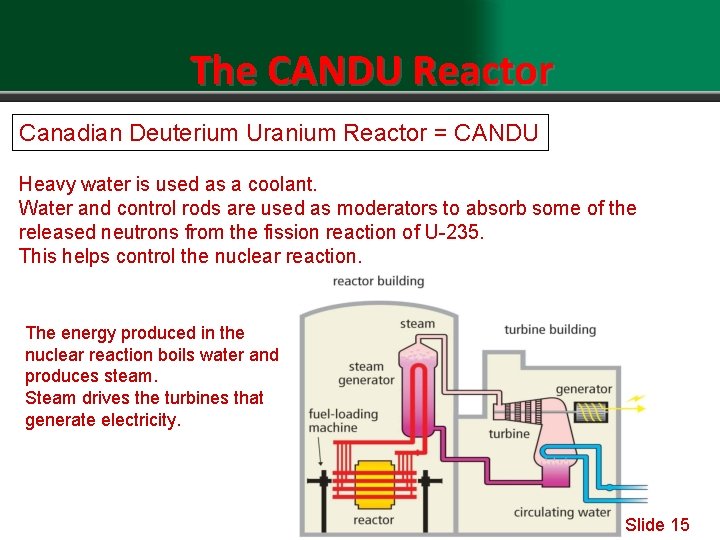
The CANDU Reactor Canadian Deuterium Uranium Reactor = CANDU Heavy is used as a coolant. Lightwater = H 2 O Water and control rods are used as moderators to absorb some of the Heavy water uses deuterium or a heavy Hydrogen isotope released neutrons from the fission reaction of U-235. This helps control the nuclear reaction. The energy produced in the nuclear reaction boils water and produces steam. Steam drives the turbines that generate electricity. Slide 15

Nuclear Reactors Light water = H 2 O Heavy water uses deuterium or a heavy Hydrogen isotope Slide 16

Nuclear Reactors Light water = H 2 O Heavy water uses deuterium or a heavy Hydrogen isotope Slide 17

Nuclear Fusion • Nuclear fusion = joining of two light nuclei into one heavier nucleus. • In the core of the Sun, two hydrogen nuclei join under tremendous heat and pressure to form a helium nucleus • • When the helium atom is formed, huge amounts of energy are released Requires about 106 C This is the reaction used This is the reaction: in a hydrogen bomb Energy FUSION BETWEEN HYDROGEN-2 (deuterium) AND HYDROGEN-3 (tritium) Notice the product is bigger than the reactants Slide 18

Nuclear Fusion Slide 19
Nuclear Fusion Slide 20

Nuclear Fusion Slide 21

Atom Bombs vs. Hydrogen Bombs • Atom bombs (conventional nuclear bombs) release energy by fissioning (breaking apart heavy atomic nuclei like uranium and plutonium. • Hydrogen bombs release energy by fusing together light nuclei like tritium and deuterium. • This converts even more matter into energy. • A fusion bomb is detonated first to create enough heat (about 20 million degrees F) to allow for fusion of light nuclei into heavier elements. Slide 22
- Slides: 25