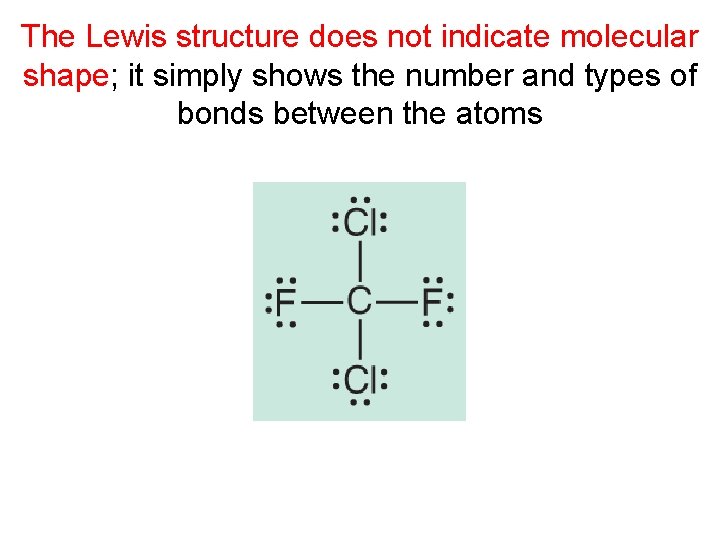
The Lewis structure does not indicate molecular shape





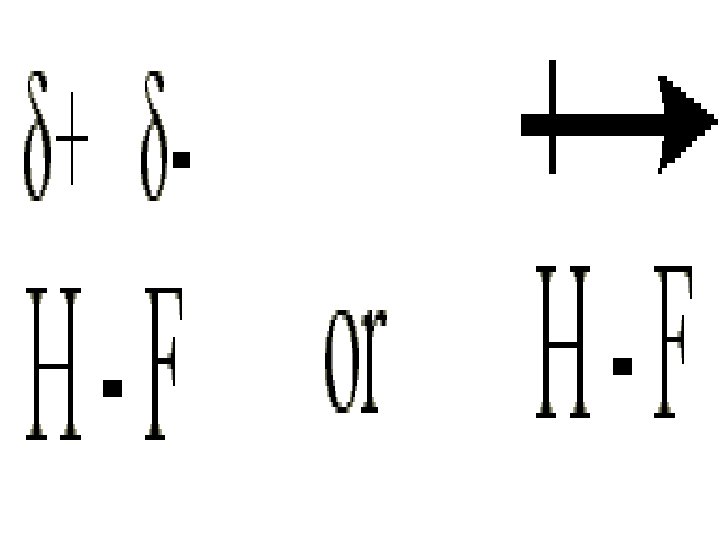

- Slides: 39

The Lewis structure does not indicate molecular shape; it simply shows the number and types of bonds between the atoms

http: //a 3. vox. com/6 a 00 b 8 ea 071 cde 1 bc 000 c 22526 e 35 b 604 a-320 pi

http: //www. billybear 4 kids. com/paperdoll/Back 2 School/Paper. Doll 02. gif


The size and shape of a molecule help determine the properties of that molecule • The nose can differentiate between thousands of odors, but do you know how the nose knows? Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.

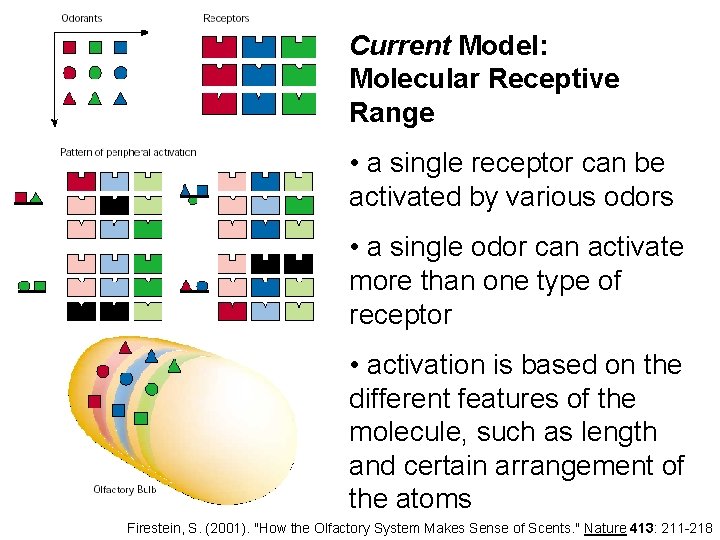
Current Model: Molecular Receptive Range • a single receptor can be activated by various odors • a single odor can activate more than one type of receptor • activation is based on the different features of the molecule, such as length and certain arrangement of the atoms Firestein, S. (2001). "How the Olfactory System Makes Sense of Scents. " Nature 413: 211 -218

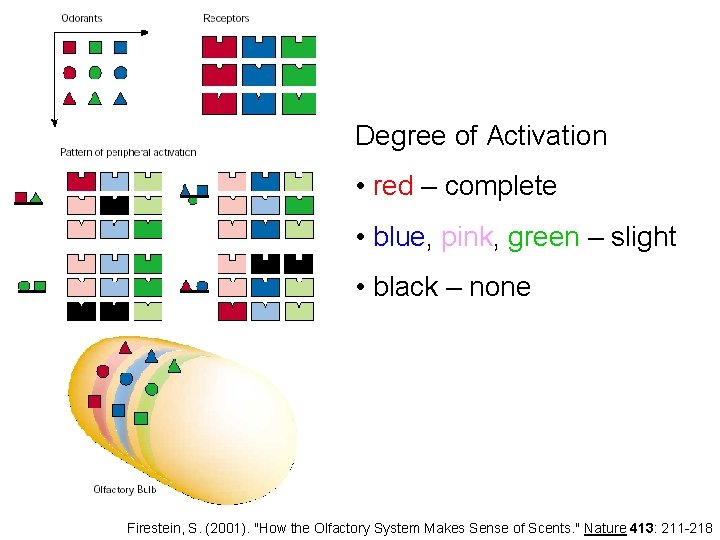
Degree of Activation • red – complete • blue, pink, green – slight • black – none Firestein, S. (2001). "How the Olfactory System Makes Sense of Scents. " Nature 413: 211 -218

Valence-Shell Electron-Pair Repulsion (VSEPR) theory

Opposites Attract attract

Opposites Attract repel

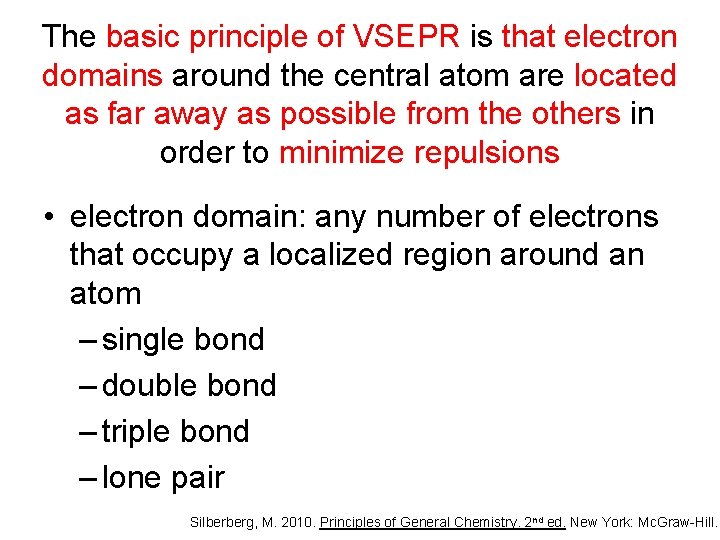
The basic principle of VSEPR is that electron domains around the central atom are located as far away as possible from the others in order to minimize repulsions • electron domain: any number of electrons that occupy a localized region around an atom – single bond – double bond – triple bond – lone pair Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

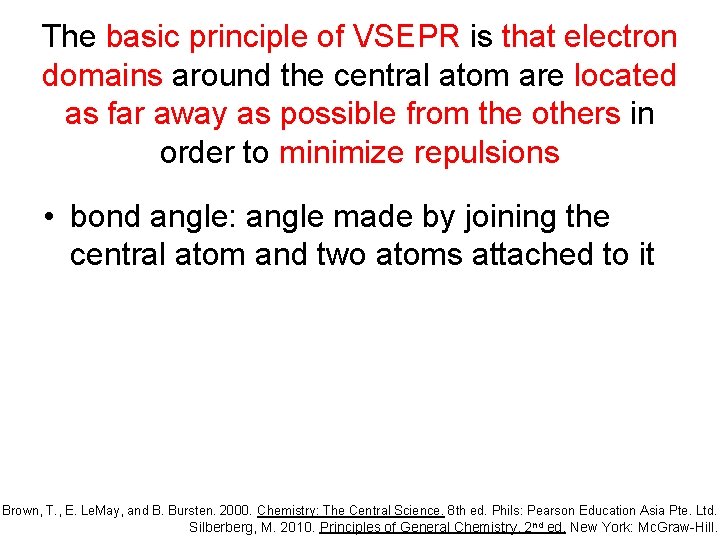
The basic principle of VSEPR is that electron domains around the central atom are located as far away as possible from the others in order to minimize repulsions • bond angle: angle made by joining the central atom and two atoms attached to it Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

Electron domain geometry: Defined by the electron domains around the central atom

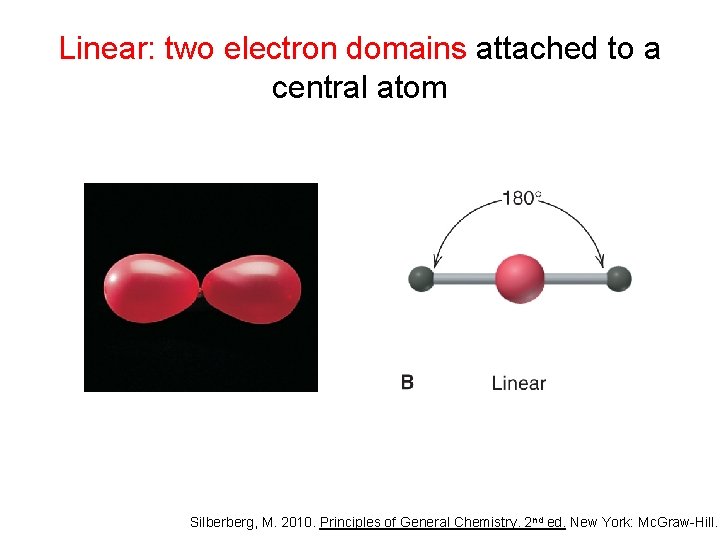
Linear: two electron domains attached to a central atom Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

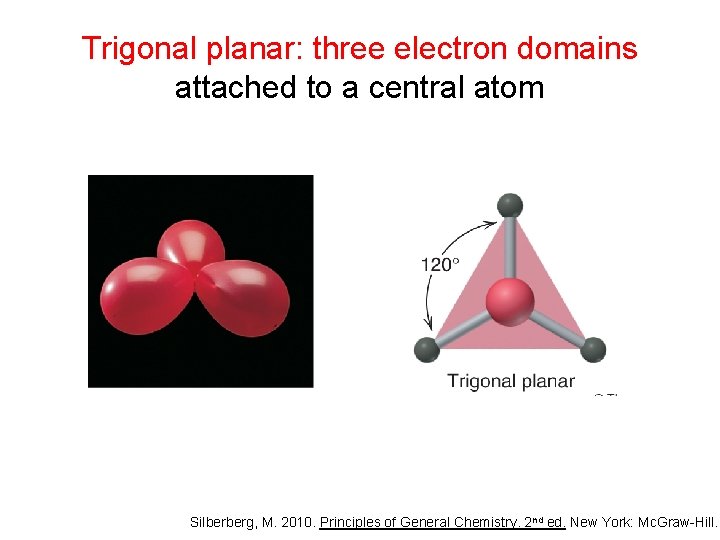
Trigonal planar: three electron domains attached to a central atom Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

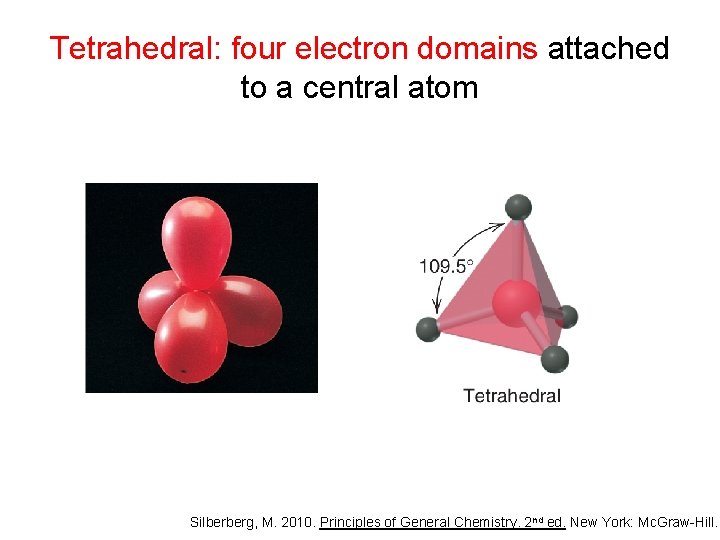
Tetrahedral: four electron domains attached to a central atom Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

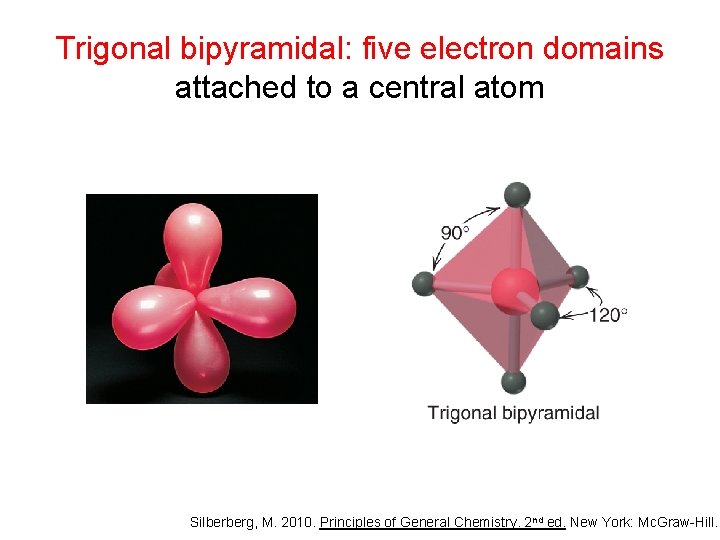
Trigonal bipyramidal: five electron domains attached to a central atom Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

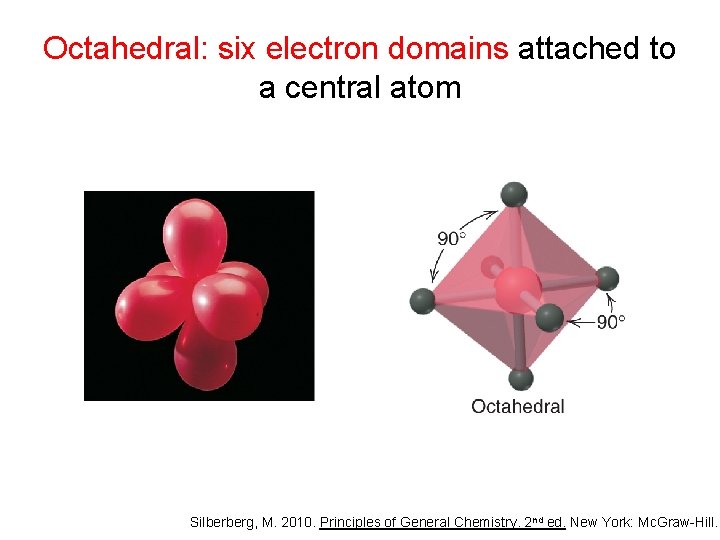
Octahedral: six electron domains attached to a central atom Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

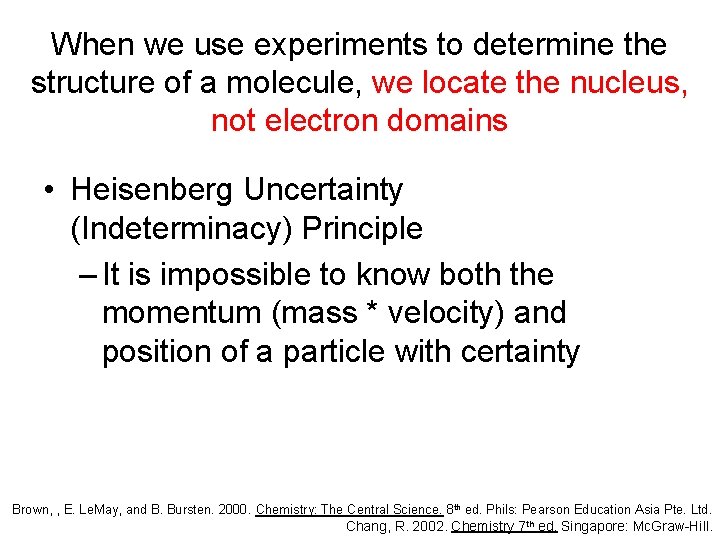
When we use experiments to determine the structure of a molecule, we locate the nucleus, not electron domains • Heisenberg Uncertainty (Indeterminacy) Principle – It is impossible to know both the momentum (mass * velocity) and position of a particle with certainty Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.

Molecular Geometry: Defined by the relative positions of the nucleus of the atoms

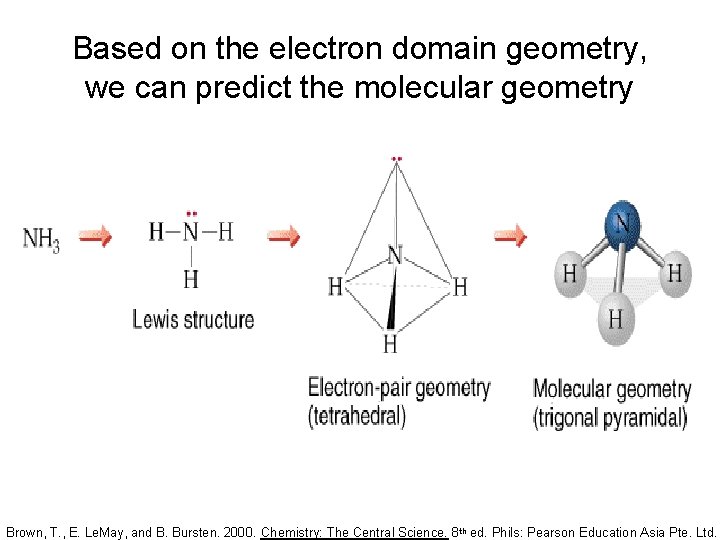
Based on the electron domain geometry, we can predict the molecular geometry Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.

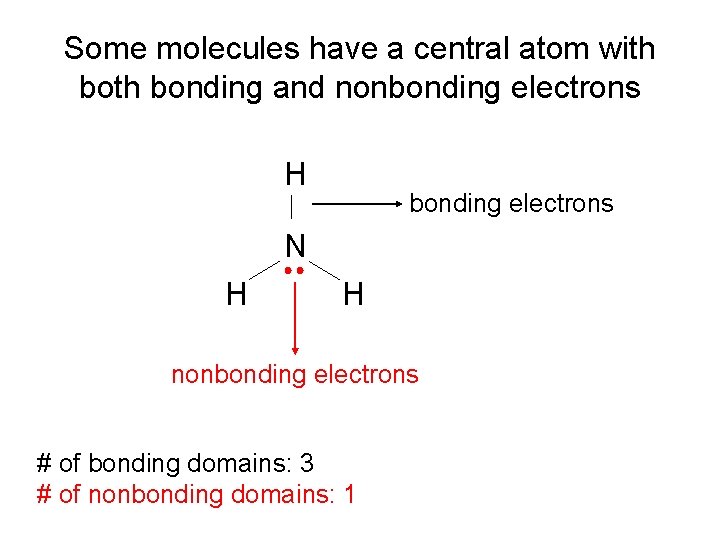
Some molecules have a central atom with bonding and nonbonding electrons H bonding electrons N H H nonbonding electrons # of bonding domains: 3 # of nonbonding domains: 1

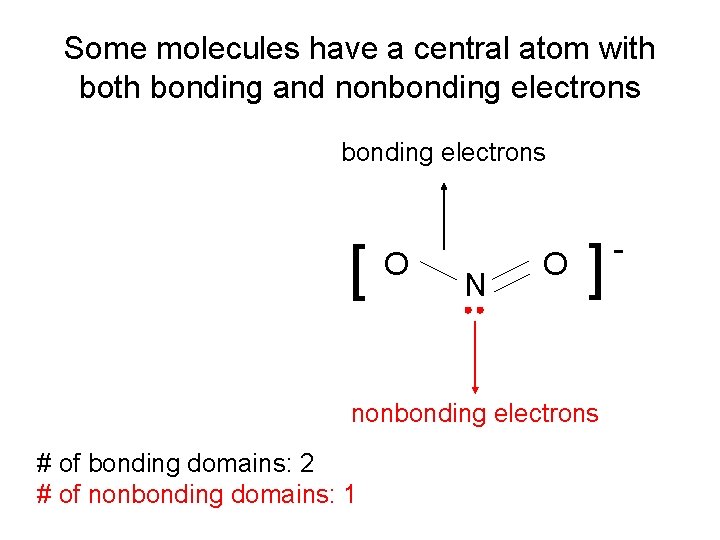
Some molecules have a central atom with bonding and nonbonding electrons N O [ [ O nonbonding electrons # of bonding domains: 2 # of nonbonding domains: 1 -

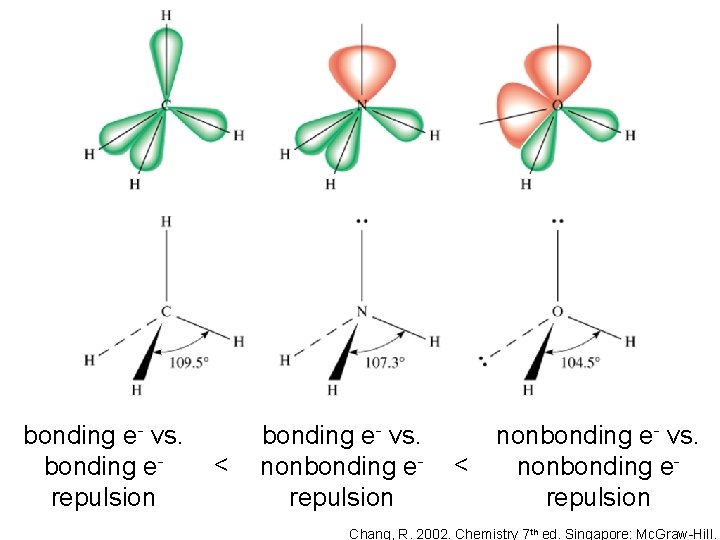
Since a lone pair is held by only one nucleus, it is less confined and exerts greater repulsions on nearby bonding pairs. As a result, the bond angle is compressed Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

bonding e- vs. bonding erepulsion < bonding e- vs. nonbonding erepulsion < nonbonding e- vs. nonbonding erepulsion Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.

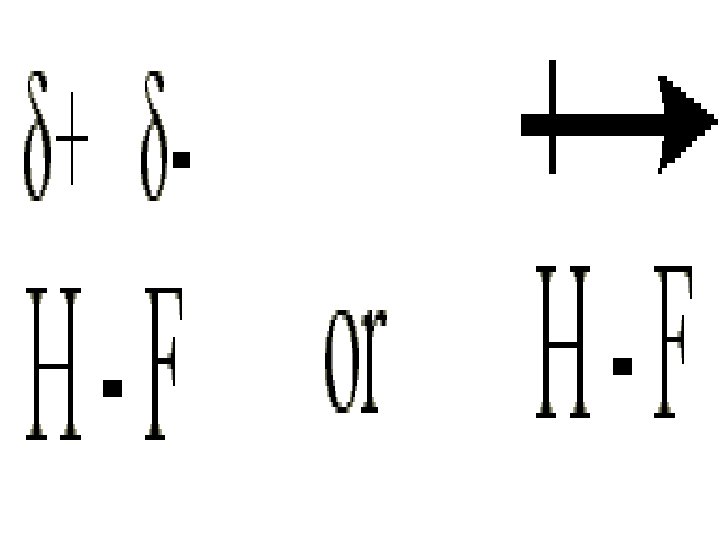



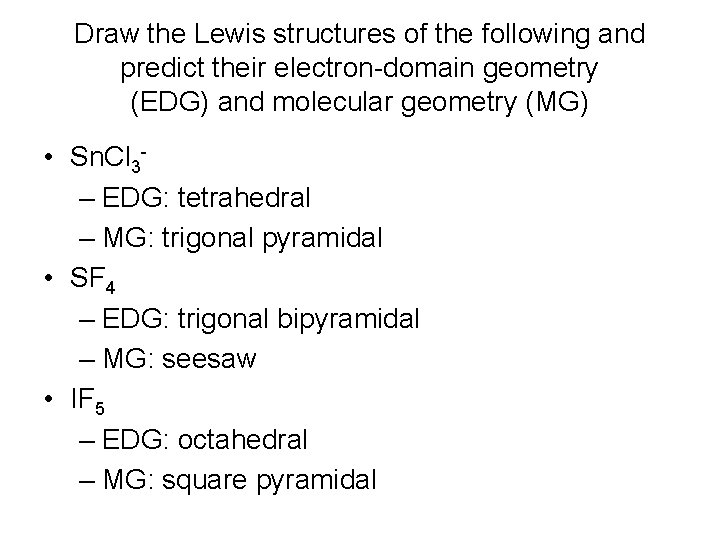
Draw the Lewis structures of the following and predict their electron-domain geometry (EDG) and molecular geometry (MG) • Sn. Cl 3– EDG: tetrahedral – MG: trigonal pyramidal • SF 4 – EDG: trigonal bipyramidal – MG: seesaw • IF 5 – EDG: octahedral – MG: square pyramidal

Polarity of molecules

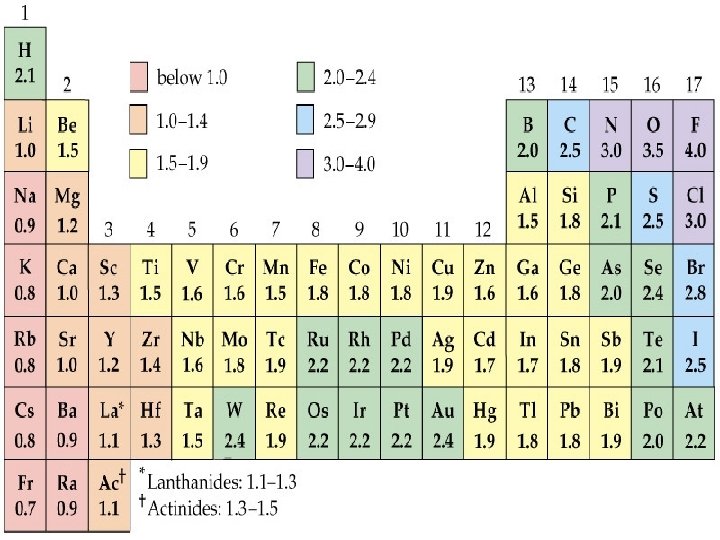
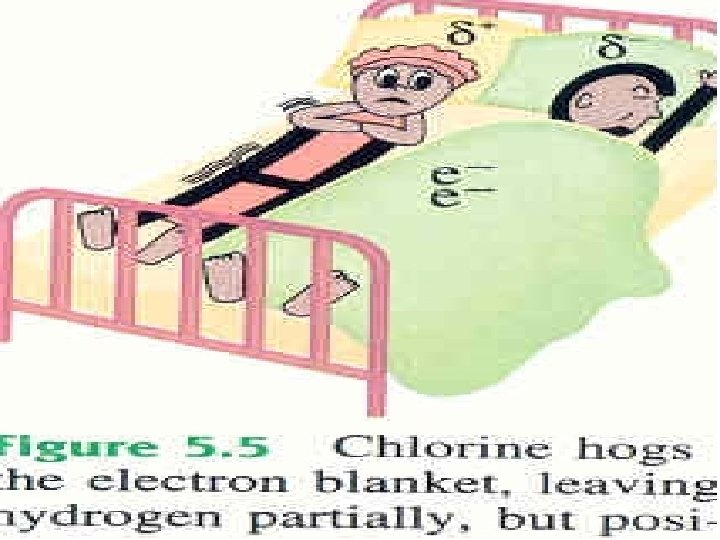
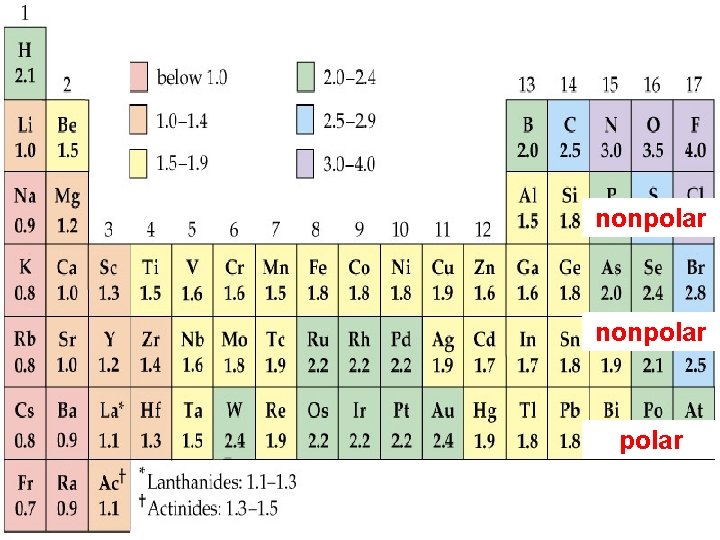
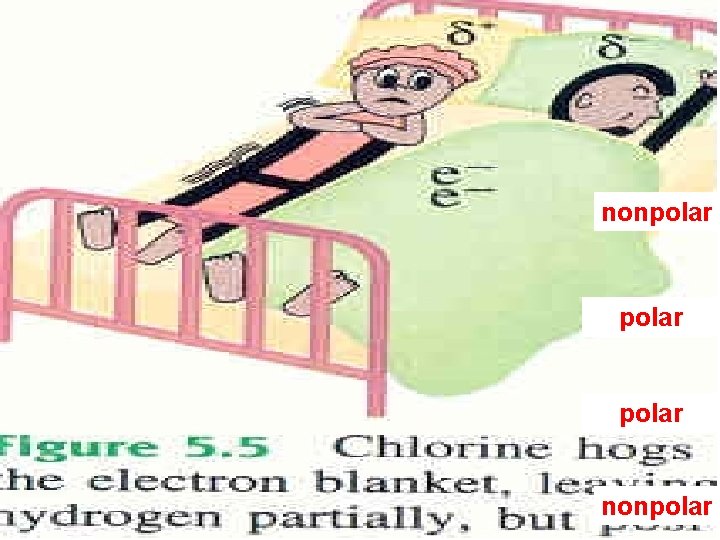
Electronegativity is the relative ability of a bonded atom to attract the shared electrons *the higher the EN of an atom, the greater the ability to pull electrons towards itself Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

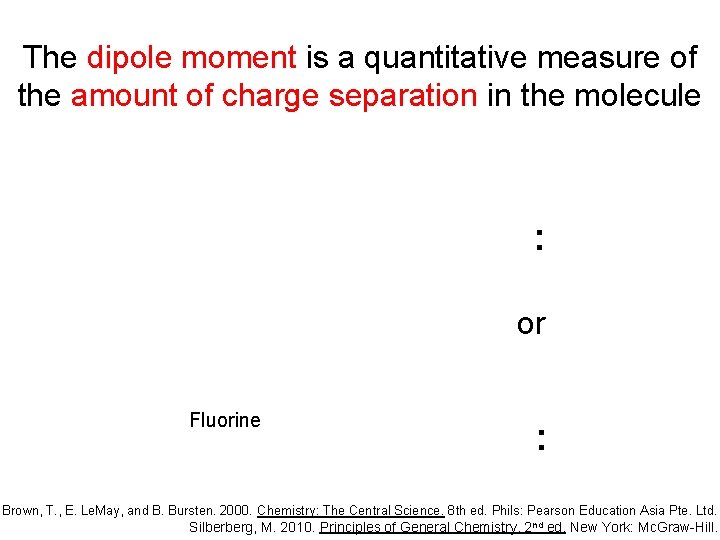
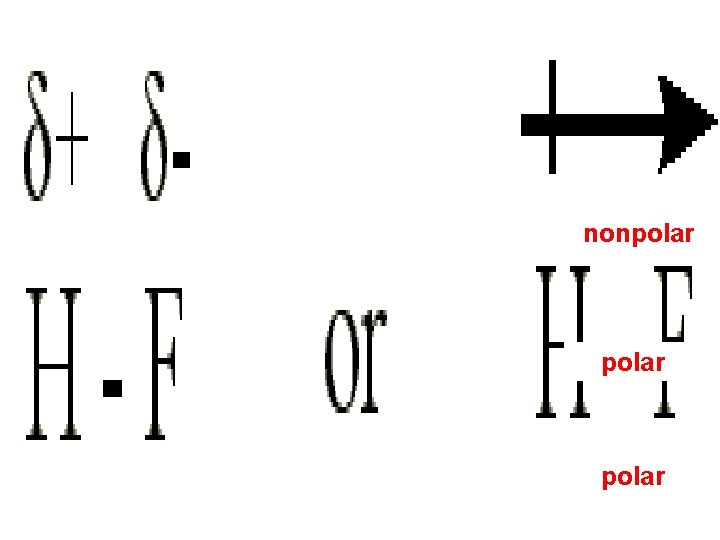
The dipole moment is a quantitative measure of the amount of charge separation in the molecule : or Fluorine : Brown, T. , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.

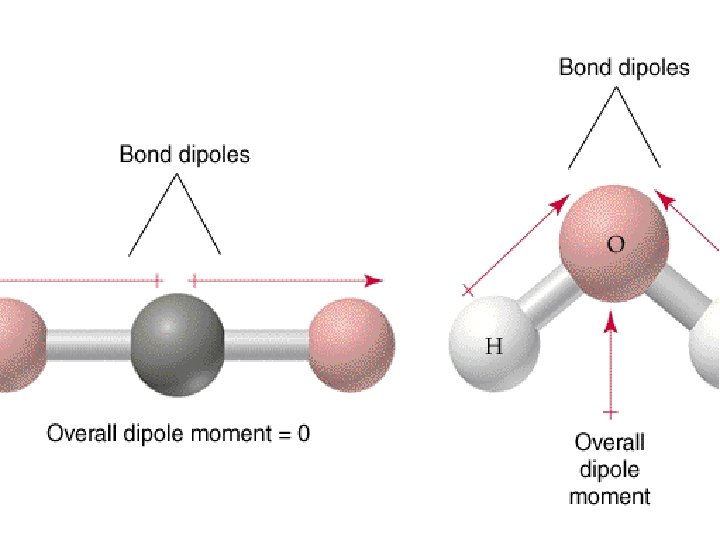
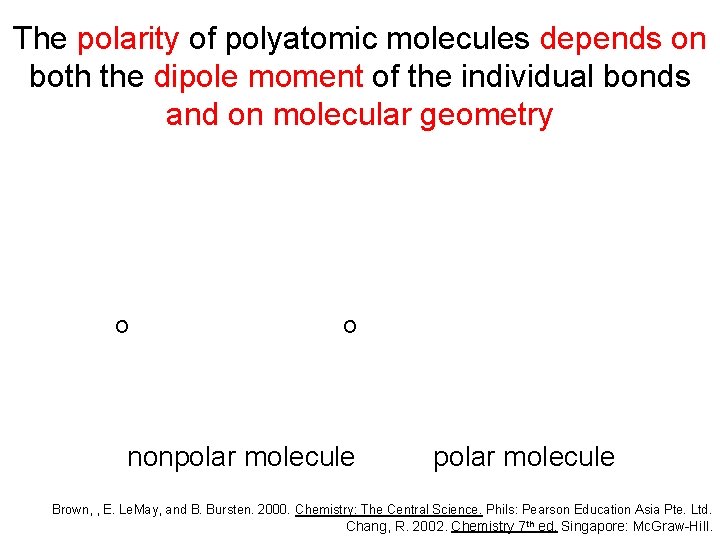
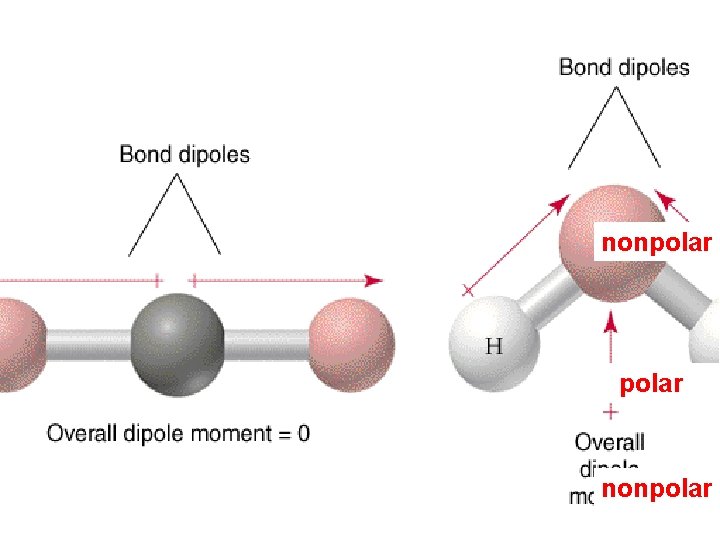
The polarity of polyatomic molecules depends on both the dipole moment of the individual bonds and on molecular geometry O C O nonpolar molecule Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.

Polarity of ABn molecules

nonpolar

nonpolar

nonpolar nonpolar

nonpolar