THE LAW OF CONSERVATION OF MATTER HYDROGEN ATOMS


- Slides: 17

THE LAW OF CONSERVATION OF MATTER

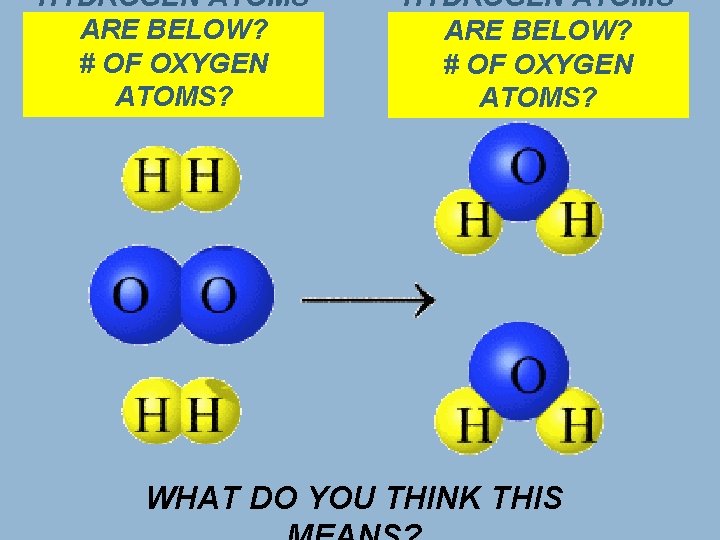
HYDROGEN ATOMS ARE BELOW? # OF OXYGEN ATOMS? WHAT DO YOU THINK THIS

The Law of Conservation of Matter states that matter cannot be created or destroyed, it can only change forms.

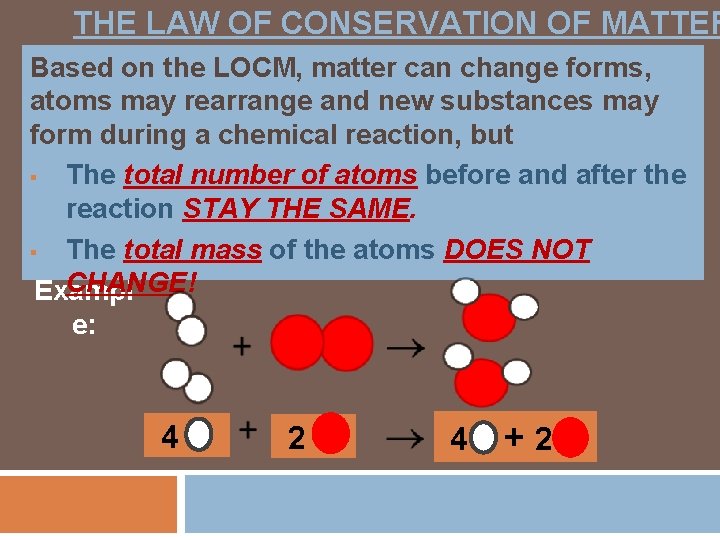
THE LAW OF CONSERVATION OF MATTER Based on the LOCM, matter can change forms, atoms may rearrange and new substances may form during a chemical reaction, but § The total number of atoms before and after the reaction STAY THE SAME. § The total mass of the atoms DOES NOT CHANGE! Exampl e: 4 2 4 +2

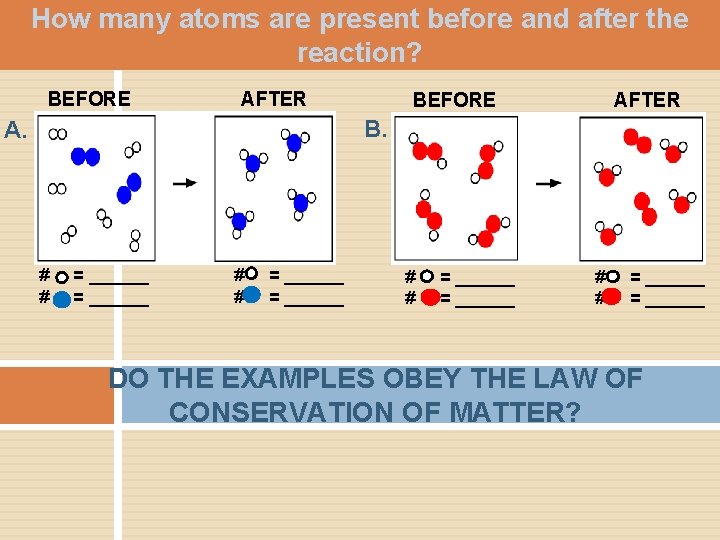
How many atoms are present before and after the reaction? BEFORE AFTER B. A. # # = ______ = ______ DO THE EXAMPLES OBEY THE LAW OF CONSERVATION OF MATTER?

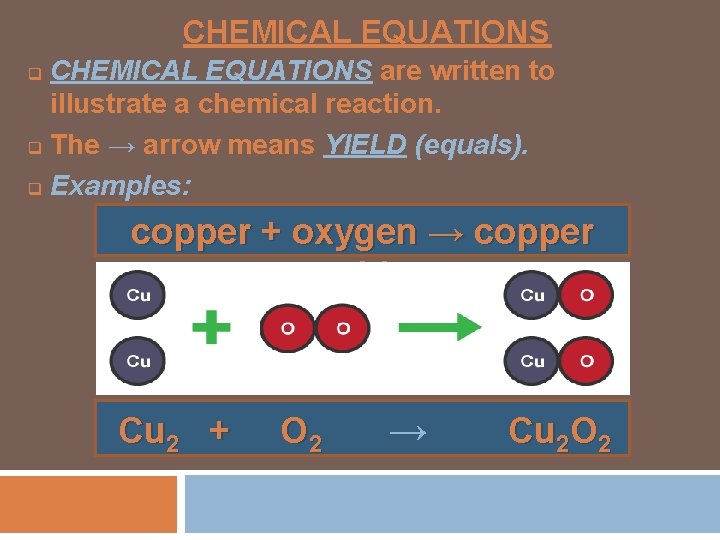
CHEMICAL EQUATIONS are written to illustrate a chemical reaction. q The → arrow means YIELD (equals). q Examples: q copper + oxygen → copper oxide Cu 2 + O 2 → Cu 2 O 2

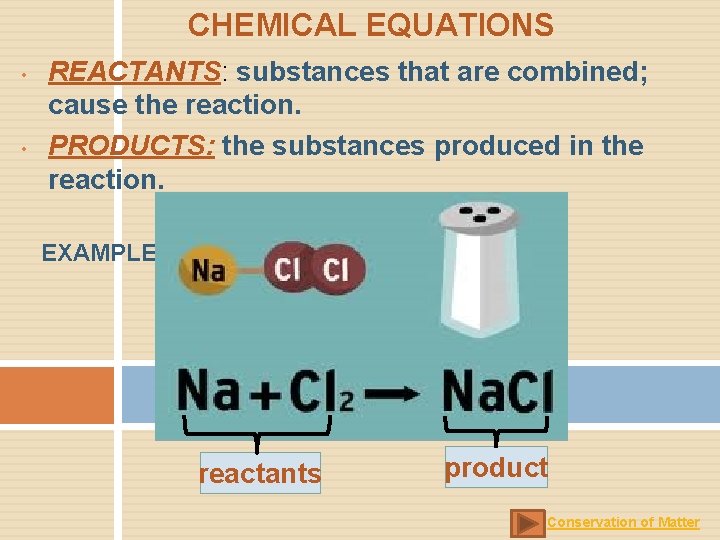
CHEMICAL EQUATIONS • • REACTANTS: substances that are combined; cause the reaction. PRODUCTS: the substances produced in the reaction. EXAMPLE: reactants product Conservation of Matter

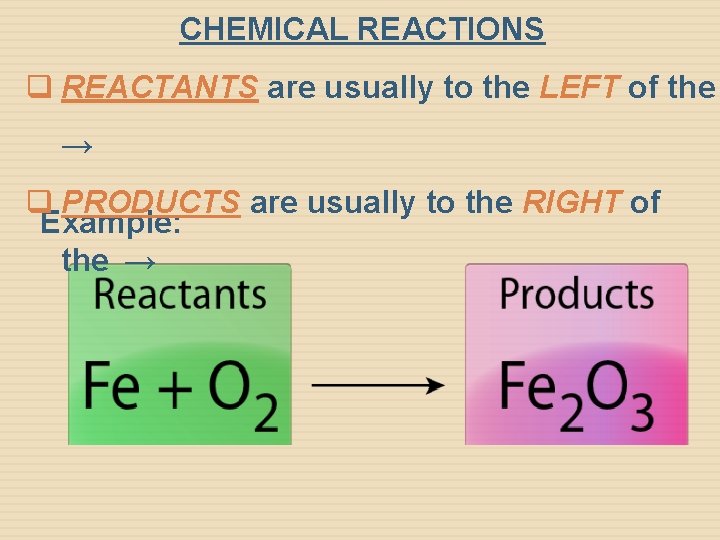
CHEMICAL REACTIONS q REACTANTS are usually to the LEFT of the → q PRODUCTS are usually to the RIGHT of Example: the →

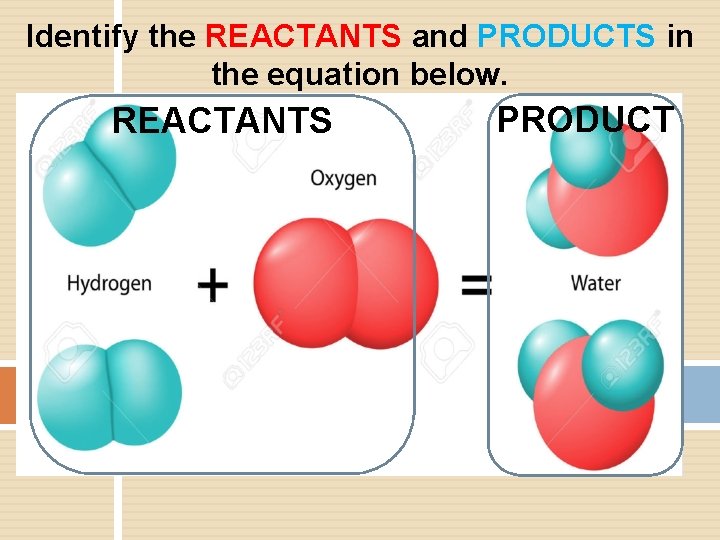
Identify the REACTANTS and PRODUCTS in the equation below. REACTANTS PRODUCT

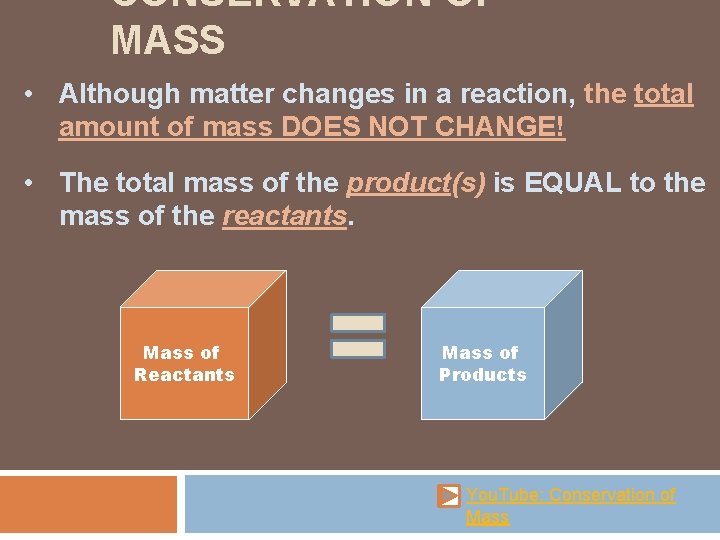
CONSERVATION OF MASS • Although matter changes in a reaction, the total amount of mass DOES NOT CHANGE! • The total mass of the product(s) is EQUAL to the mass of the reactants. Mass of Reactants Mass of Products You. Tube: Conservation of Mass

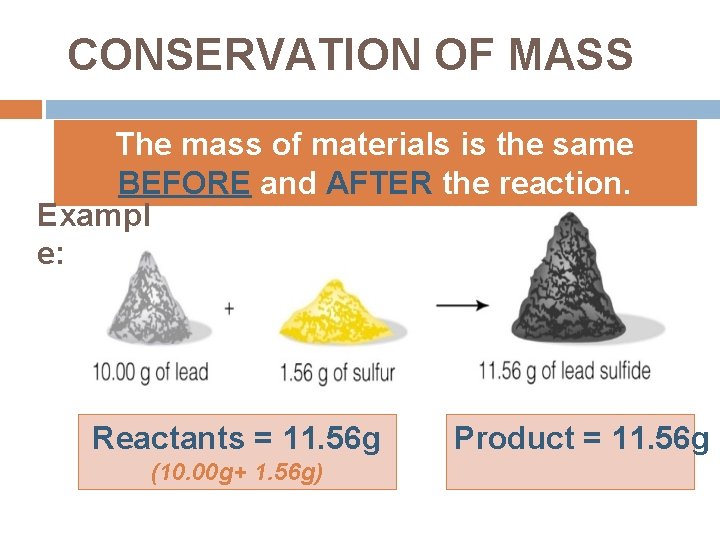
CONSERVATION OF MASS The mass of materials is the same BEFORE and AFTER the reaction. Exampl e: Reactants = 11. 56 g (10. 00 g+ 1. 56 g) Product = 11. 56 g

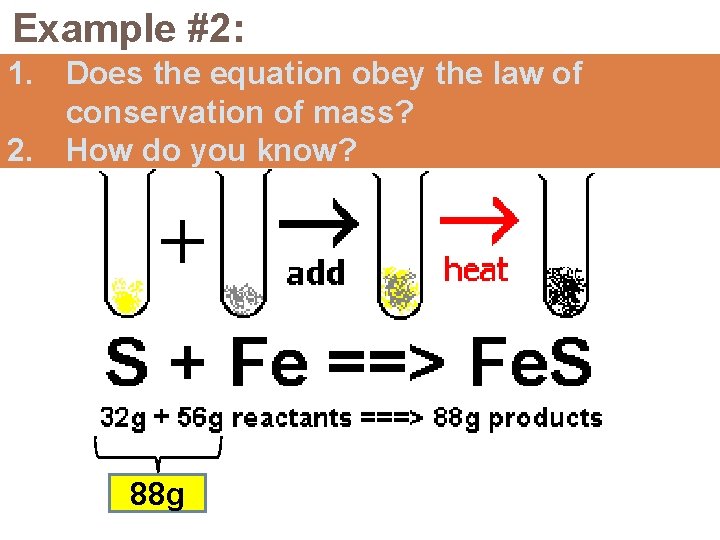
Example #2: 1. Does the equation obey the law of conservation of mass? 2. How do you know? 88 g

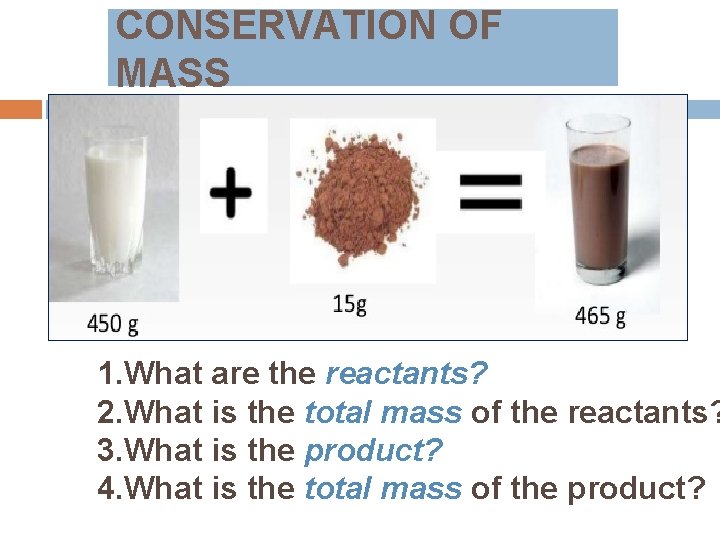
CONSERVATION OF MASS 1. What are the reactants? 2. What is the total mass of the reactants? 3. What is the product? 4. What is the total mass of the product?

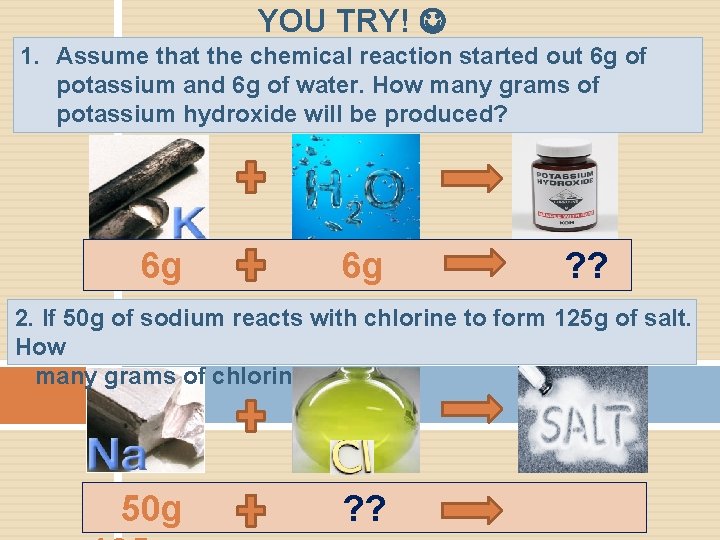
YOU TRY! 1. Assume that the chemical reaction started out 6 g of potassium and 6 g of water. How many grams of potassium hydroxide will be produced? 6 g 6 g ? ? 2. If 50 g of sodium reacts with chlorine to form 125 g of salt. How many grams of chlorine reacted? 50 g ? ?

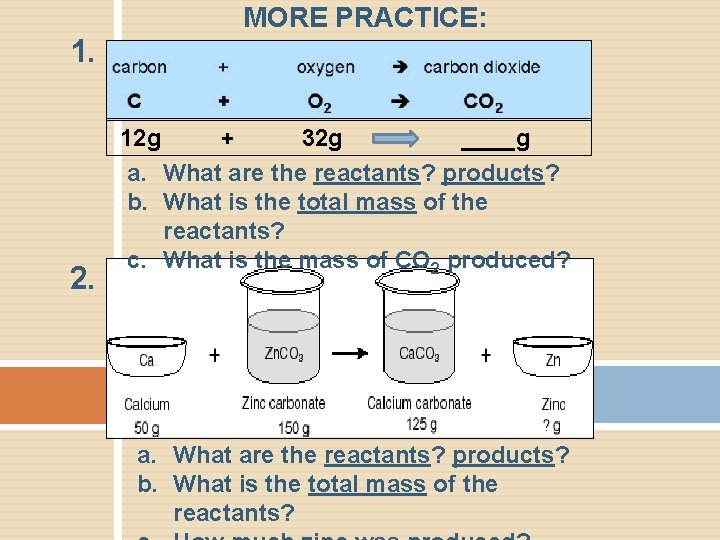
MORE PRACTICE: 1. 2. 12 g + 32 g ____g a. What are the reactants? products? b. What is the total mass of the reactants? c. What is the mass of CO 2 produced? a. What are the reactants? products? b. What is the total mass of the reactants?

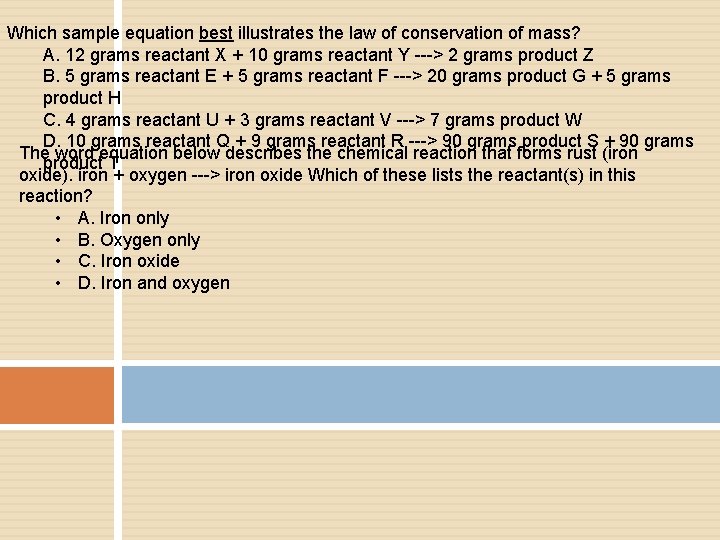
Which sample equation best illustrates the law of conservation of mass? A. 12 grams reactant X + 10 grams reactant Y ---> 2 grams product Z B. 5 grams reactant E + 5 grams reactant F ---> 20 grams product G + 5 grams product H C. 4 grams reactant U + 3 grams reactant V ---> 7 grams product W D. 10 grams reactant Q + 9 grams reactant R ---> 90 grams product S + 90 grams The word equation below describes the chemical reaction that forms rust (iron product T oxide). iron + oxygen ---> iron oxide Which of these lists the reactant(s) in this reaction? • A. Iron only • B. Oxygen only • C. Iron oxide • D. Iron and oxygen

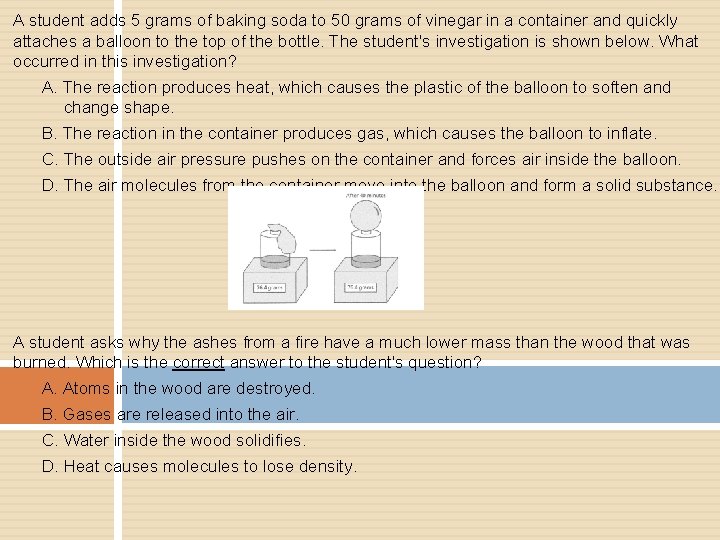
A student adds 5 grams of baking soda to 50 grams of vinegar in a container and quickly attaches a balloon to the top of the bottle. The student's investigation is shown below. What occurred in this investigation? A. The reaction produces heat, which causes the plastic of the balloon to soften and change shape. B. The reaction in the container produces gas, which causes the balloon to inflate. C. The outside air pressure pushes on the container and forces air inside the balloon. D. The air molecules from the container move into the balloon and form a solid substance. A student asks why the ashes from a fire have a much lower mass than the wood that was burned. Which is the correct answer to the student's question? A. Atoms in the wood are destroyed. B. Gases are released into the air. C. Water inside the wood solidifies. D. Heat causes molecules to lose density.