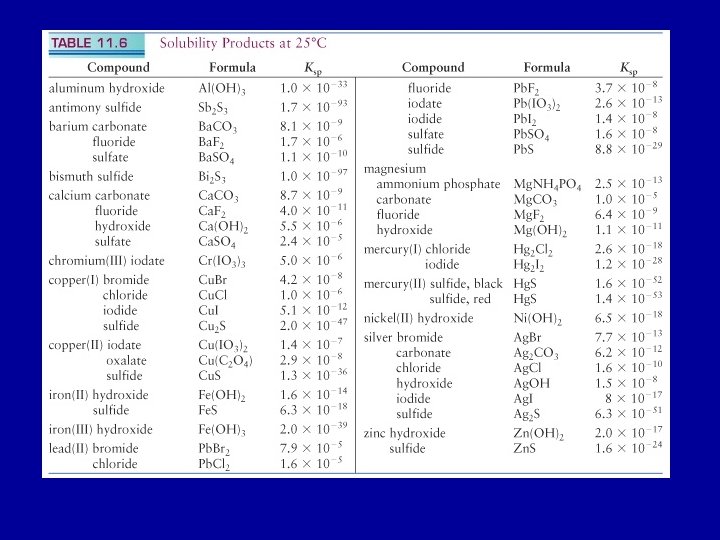
The Ksp of chromium III iodate in water

![Ksp = [Pb 2+ (aq)] [I- (aq)]2 If Q > Ksp precipitation; if Q Ksp = [Pb 2+ (aq)] [I- (aq)]2 If Q > Ksp precipitation; if Q](https://slidetodoc.com/presentation_image_h2/3ba4f34d7cd34a945265585b56dfca71/image-3.jpg)




- Slides: 19

The Ksp of chromium (III) iodate in water is 5. 0 x 10 -6. Estimate the molar solubility of the compound. Cr(IO 3)3(s) Cr 3+(aq) + 3 IO 3 -(aq) 1 mole Cr(IO 3)3 produces 1 mole Cr 3+ and 3 moles IO 3 Ksp = [Cr 3+(aq)] [IO 3 -(aq)3 = (s) (3 s)3 = 5. 0 x 10 -6 where s is the solubility of Cr(IO 3)3 s = 0. 021 Molar solubility Cr(IO 3)3 of 0. 021 M

Precipitation from Solution If equal volumes of aqueous solutions of 0. 2 M Pb(NO 3)2 and KI are mixed will Pb. I 2(s) precipitate out? Ksp of Pb. I 2 is 1. 4 x 10 -8 Use the reaction quotient, Q, to predict whether precipitation will occur Pb(NO 3)2 (aq) +2 KI(aq) -> Pb. I 2 (s) + 2 KNO 3 (aq) Net ionic equation: Pb 2+ (aq) + 2 I- (aq) -> Pb. I 2 (s) The reverse of this reaction defines Ksp Pb. I 2 (s) Pb 2+ (aq) + 2 I- (aq)
![Ksp Pb 2 aq I aq2 If Q Ksp precipitation if Q Ksp = [Pb 2+ (aq)] [I- (aq)]2 If Q > Ksp precipitation; if Q](https://slidetodoc.com/presentation_image_h2/3ba4f34d7cd34a945265585b56dfca71/image-3.jpg)
Ksp = [Pb 2+ (aq)] [I- (aq)]2 If Q > Ksp precipitation; if Q < Ksp no precipitation Equal volumes of Pb(NO 3)2 and KI are mixed On mixing, volume of mixed solution is twice initial volume [Pb 2+ (aq)] = 0. 2 M / 2 = 0. 1 M [I- (aq)] = 0. 1 M Q = [Pb 2+(aq)] [I- (aq)]2 = (0. 1)2 = 0. 001 M Q > Ksp; Pb. I 2(s) precipitates

Common Ion Effect Adding Na. Cl to a saturated solution of Ag. Cl lowers the solubility of Ag. Cl, reducing the amount of Ag+(aq) and - (aq) Cl Ag. Cl(s) Ag+(aq) + Cl-(aq) The common ion effect is the reduction in the solubility of a sparingly soluble salt by the addition of a soluble salt that has an ion in common with it. Example of Le. Chatelier’s principle.

Ag. Cl(s) Ag+(aq) + Cl- (aq) Ksp = 1. 6 x 10 -10 at 25 o. C [Ag+ (aq)] = [Cl- (aq)] = 1. 3 x 10 -5 M concentration of dissolved Ag. Cl = 1. 3 x 10 -5 M Dissolve Ag. Cl in a solution of 0. 10 M Na. Cl. What is the solubility of Ag. Cl in the Na. Cl solution? [Cl-(aq)] = 0. 10 M Since Ksp at 25 o. C is a constant, [Ag+(aq)] = Ksp / [Cl- (aq)] = 1. 6 x 10 -9 M Concentration of dissolved Ag. Cl = 1. 6 x 10 -9 M

Selective Precipitation A mixture of cations in solution can be separated by adding anions with which they form salts with different solubilities. A few drops of a solution of Pb(NO 3)2(aq) is added to a solution of KI(aq), yellow Pb. I 2(s) is formed


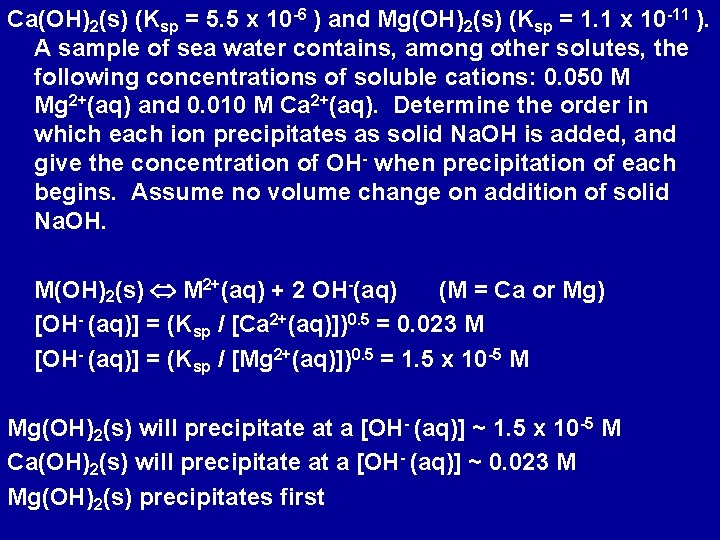
Ca(OH)2(s) (Ksp = 5. 5 x 10 -6 ) and Mg(OH)2(s) (Ksp = 1. 1 x 10 -11 ). A sample of sea water contains, among other solutes, the following concentrations of soluble cations: 0. 050 M Mg 2+(aq) and 0. 010 M Ca 2+(aq). Determine the order in which each ion precipitates as solid Na. OH is added, and give the concentration of OH- when precipitation of each begins. Assume no volume change on addition of solid Na. OH. M(OH)2(s) M 2+(aq) + 2 OH-(aq) (M = Ca or Mg) [OH- (aq)] = (Ksp / [Ca 2+(aq)])0. 5 = 0. 023 M [OH- (aq)] = (Ksp / [Mg 2+(aq)])0. 5 = 1. 5 x 10 -5 M Mg(OH)2(s) will precipitate at a [OH- (aq)] ~ 1. 5 x 10 -5 M Ca(OH)2(s) will precipitate at a [OH- (aq)] ~ 0. 023 M Mg(OH)2(s) precipitates first

Dissolving Precipitates The solubility of insoluble compounds can often be increased by addition of acids. Zn. CO 3 (s) Zn 2+ (aq) + CO 32 - (aq) Adding acid like HNO 3(aq) CO 32 - (aq) + 2 HNO 3(aq) -> CO 2 (g) + H 2 O(l) + 2 NO 3 - (aq) Addition of acids reacts with the anions in solution lowering the concentration of the anion. The insoluble compound then dissolves further to increase the concentration of the anion in solution. Another example of Le. Chatelier’s principle in action.

The solubility of a solid can be increased by removing an ion from solution. Acids can be used to dissolve hydroxides, sulfites, or carbonate precipitates. Mg(OH)2(s) Mg 2+(aq) + 2 OH-(aq) Ksp = 1. 1 x 10 -11 In acidic p. H concentration of OH- is lowered; increases solubility of the metal hydroxide

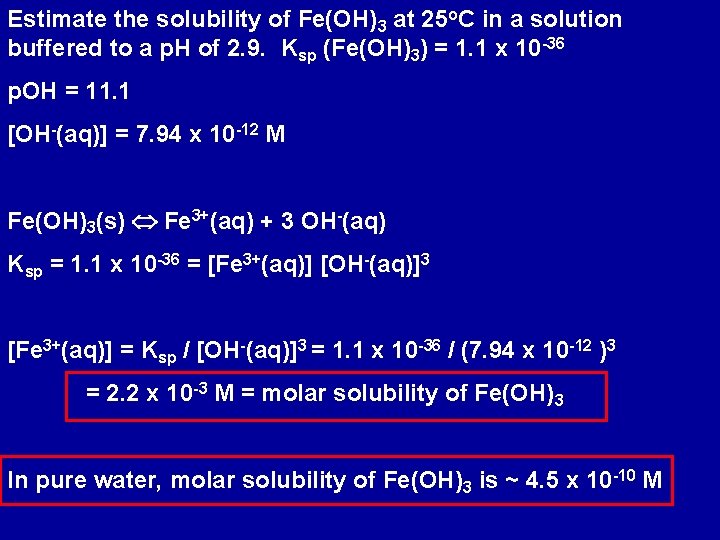
Estimate the solubility of Fe(OH)3 at 25 o. C in a solution buffered to a p. H of 2. 9. Ksp (Fe(OH)3) = 1. 1 x 10 -36 p. OH = 11. 1 [OH-(aq)] = 7. 94 x 10 -12 M Fe(OH)3(s) Fe 3+(aq) + 3 OH-(aq) Ksp = 1. 1 x 10 -36 = [Fe 3+(aq)] [OH-(aq)]3 [Fe 3+(aq)] = Ksp / [OH-(aq)]3 = 1. 1 x 10 -36 / (7. 94 x 10 -12 )3 = 2. 2 x 10 -3 M = molar solubility of Fe(OH)3 In pure water, molar solubility of Fe(OH)3 is ~ 4. 5 x 10 -10 M

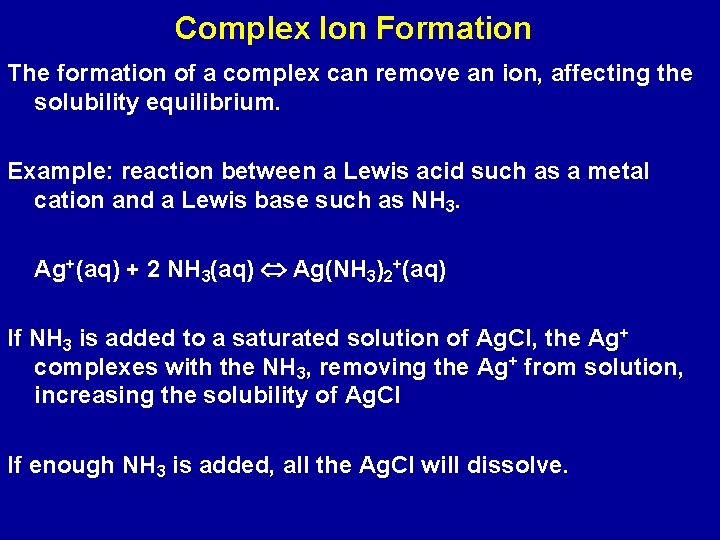
Complex Ion Formation The formation of a complex can remove an ion, affecting the solubility equilibrium. Example: reaction between a Lewis acid such as a metal cation and a Lewis base such as NH 3. Ag+(aq) + 2 NH 3(aq) Ag(NH 3)2+(aq) If NH 3 is added to a saturated solution of Ag. Cl, the Ag+ complexes with the NH 3, removing the Ag+ from solution, increasing the solubility of Ag. Cl If enough NH 3 is added, all the Ag. Cl will dissolve.

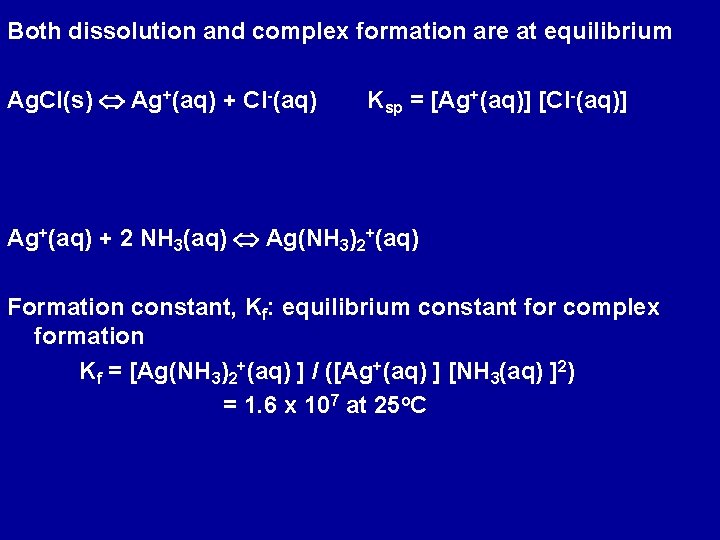
Both dissolution and complex formation are at equilibrium Ag. Cl(s) Ag+(aq) + Cl-(aq) Ksp = [Ag+(aq)] [Cl-(aq)] Ag+(aq) + 2 NH 3(aq) Ag(NH 3)2+(aq) Formation constant, Kf: equilibrium constant for complex formation Kf = [Ag(NH 3)2+(aq) ] / ([Ag+(aq) ] [NH 3(aq) ]2) = 1. 6 x 107 at 25 o. C

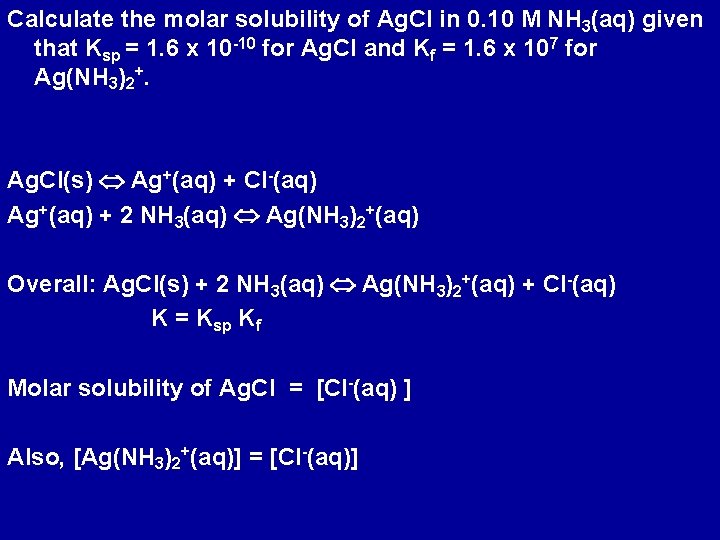
Calculate the molar solubility of Ag. Cl in 0. 10 M NH 3(aq) given that Ksp = 1. 6 x 10 -10 for Ag. Cl and Kf = 1. 6 x 107 for Ag(NH 3)2+. Ag. Cl(s) Ag+(aq) + Cl-(aq) Ag+(aq) + 2 NH 3(aq) Ag(NH 3)2+(aq) Overall: Ag. Cl(s) + 2 NH 3(aq) Ag(NH 3)2+(aq) + Cl-(aq) K = Ksp Kf Molar solubility of Ag. Cl = [Cl-(aq) ] Also, [Ag(NH 3)2+(aq)] = [Cl-(aq)]

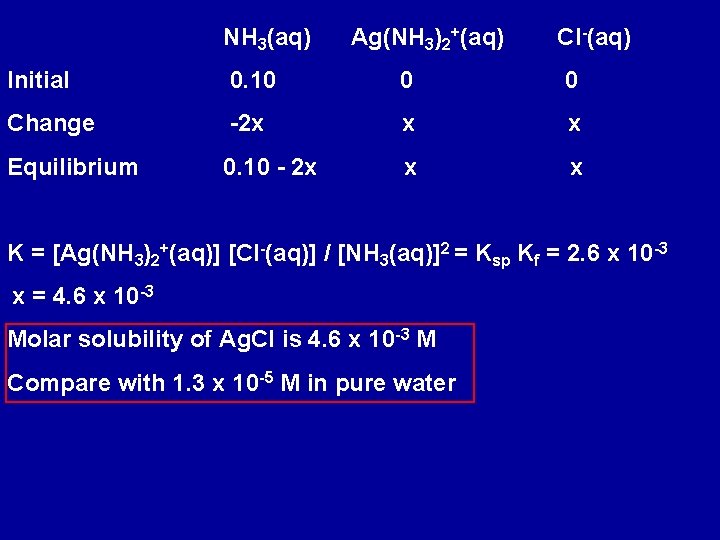
NH 3(aq) Ag(NH 3)2+(aq) Cl-(aq) Initial 0. 10 0 0 Change -2 x x x Equilibrium 0. 10 - 2 x x x K = [Ag(NH 3)2+(aq)] [Cl-(aq)] / [NH 3(aq)]2 = Ksp Kf = 2. 6 x 10 -3 x = 4. 6 x 10 -3 Molar solubility of Ag. Cl is 4. 6 x 10 -3 M Compare with 1. 3 x 10 -5 M in pure water

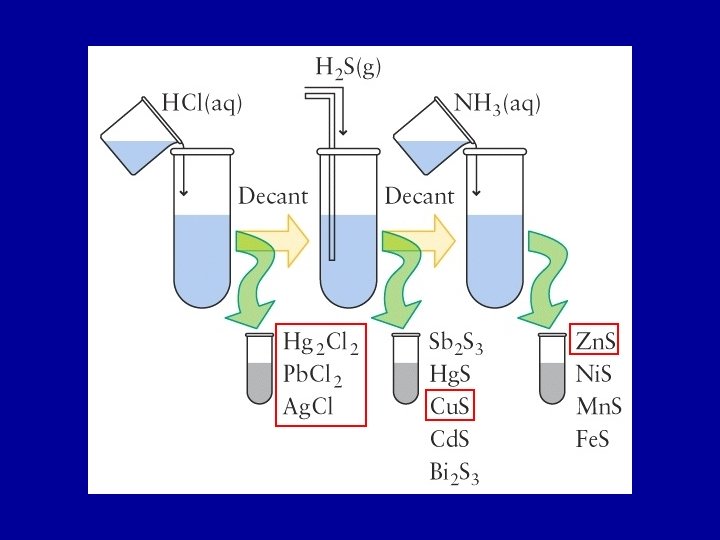
Qualitative Analysis involves the separation and identification of ions by techniques such as complex formation, selective precipitation, and control of the p. H of a solution. A solution of Pb 2+(aq), Hg 22+(aq), Ag+ (aq), Cu 2+(aq), Zn 2+(aq)


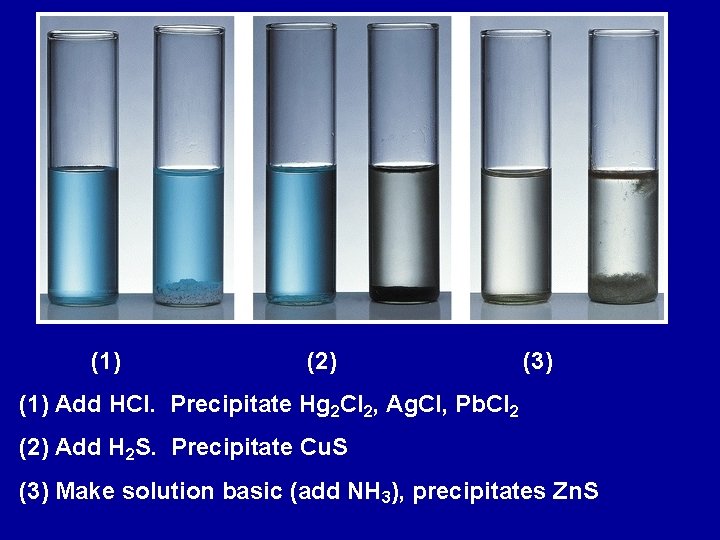
(1) (2) (3) (1) Add HCl. Precipitate Hg 2 Cl 2, Ag. Cl, Pb. Cl 2 (2) Add H 2 S. Precipitate Cu. S (3) Make solution basic (add NH 3), precipitates Zn. S

1) Precipitate of Hg 2 Cl 2, Ag. Cl, Pb. Cl 2 Rinse the precipitate in hot water; Pb. Cl 2 dissolves Add Cr. O 42 - to precipitate Pb 2+ as Pb. Cr. O 4(s) To the Hg 2 Cl 2, Ag. Cl precipitates add NH 3 to form Ag(NH 3)2+ complex which dissolves. Ag(NH 3)2+(aq) + Cl-(aq) + 2 H 3 O+(aq) Ag. Cl(s) + 2 NH 4+(aq) + 2 H 2 O(l)