The Kinetic Molecular Theory Movement in solids liquids





- Slides: 16

The Kinetic Molecular Theory Movement in solids, liquids, and gases

The theory of moving molecules! • Describes the differences between gas, liquid, and solid states.

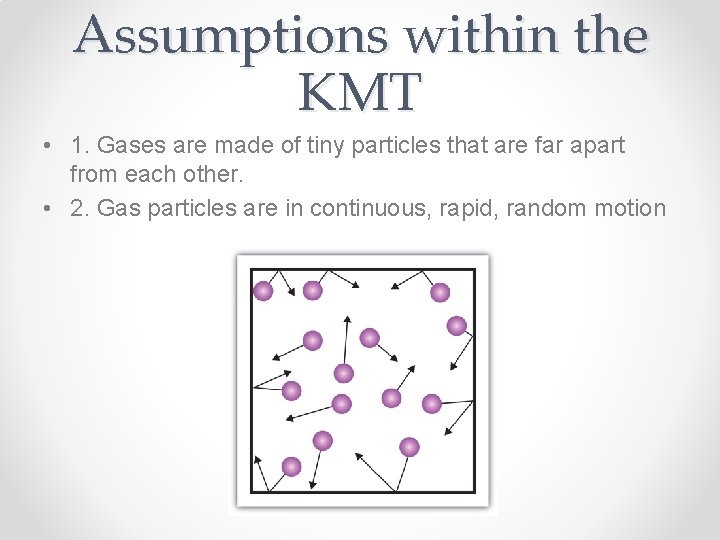
Assumptions within the KMT • 1. Gases are made of tiny particles that are far apart from each other. • 2. Gas particles are in continuous, rapid, random motion

More Assumptions • 3. There are no attractive forces between molecules under normal conditions. • 4. Collisions between particles are elastic (no energy is lost due to friction).

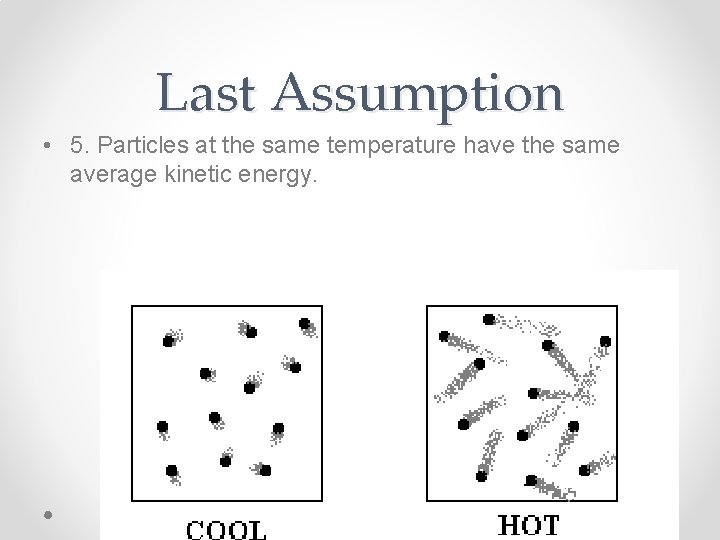
Last Assumption • 5. Particles at the same temperature have the same average kinetic energy.

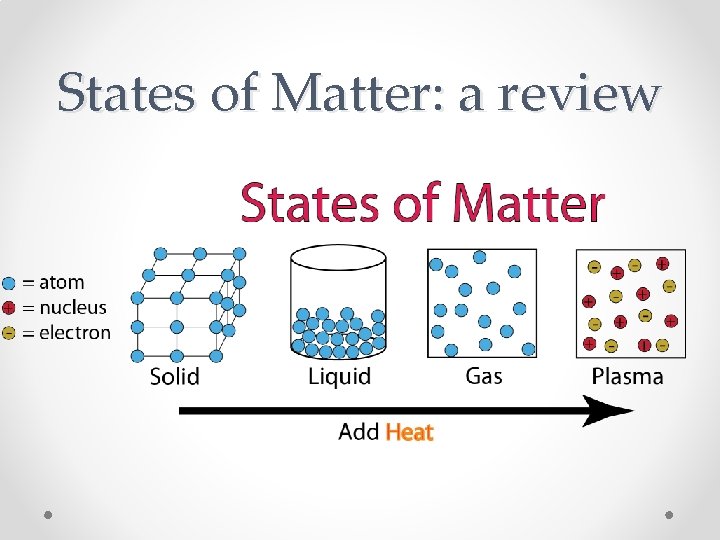
States of Matter: a review

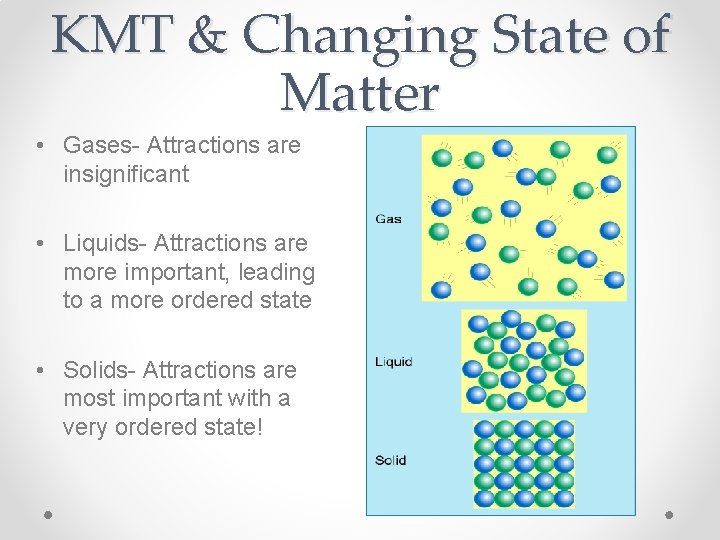
KMT & Changing State of Matter • Gases- Attractions are insignificant • Liquids- Attractions are more important, leading to a more ordered state • Solids- Attractions are most important with a very ordered state!

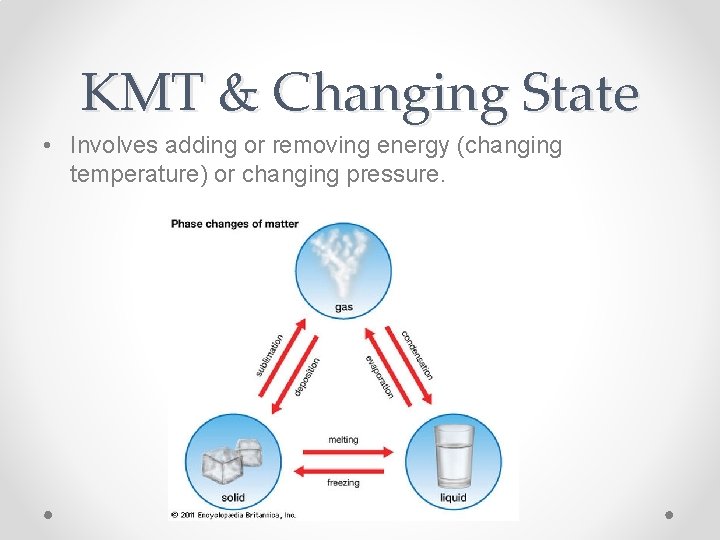
KMT & Changing State • Involves adding or removing energy (changing temperature) or changing pressure.

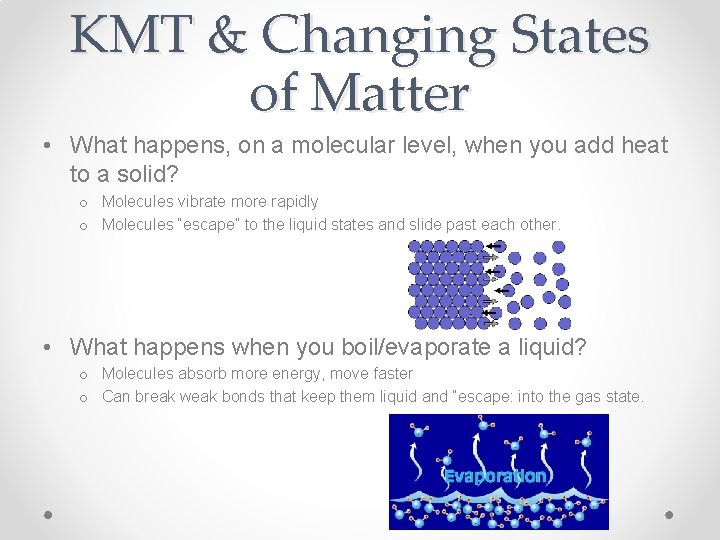
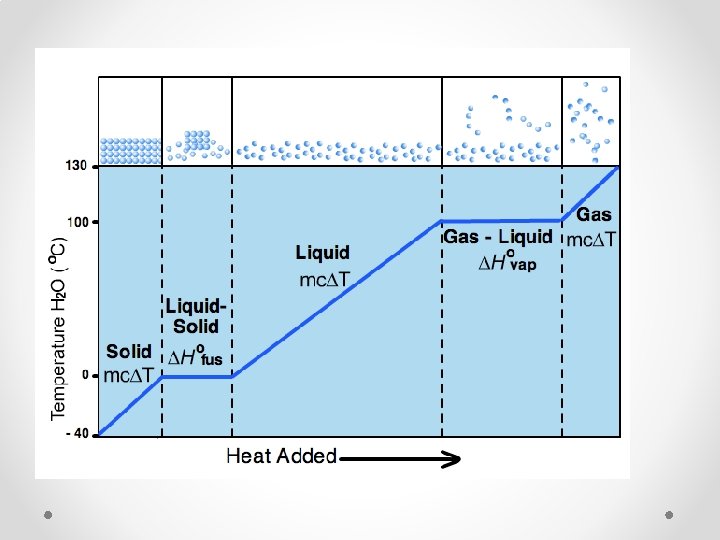
KMT & Changing States of Matter • What happens, on a molecular level, when you add heat to a solid? o Molecules vibrate more rapidly o Molecules “escape” to the liquid states and slide past each other. • What happens when you boil/evaporate a liquid? o Molecules absorb more energy, move faster o Can break weak bonds that keep them liquid and “escape: into the gas state.

KMT and Changing State of Matter • The opposite occurs when you cool a gas down until it becomes a liquid and then cool the liquid until it solidifies.

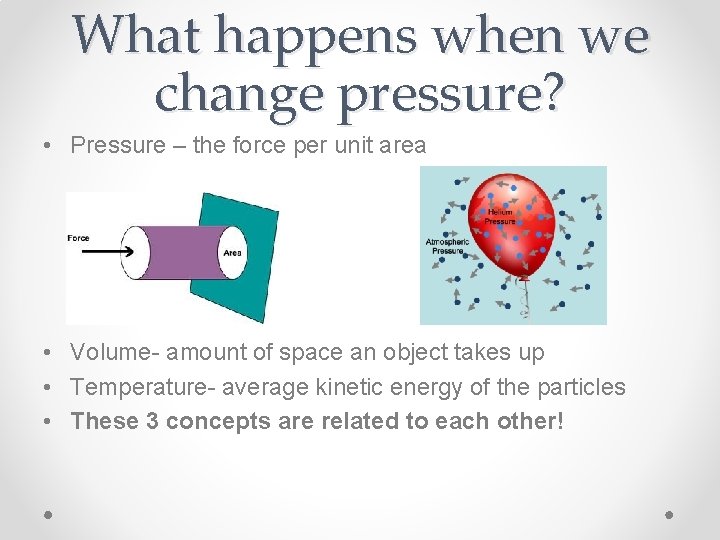
What happens when we change pressure? • Pressure – the force per unit area • Volume- amount of space an object takes up • Temperature- average kinetic energy of the particles • These 3 concepts are related to each other!

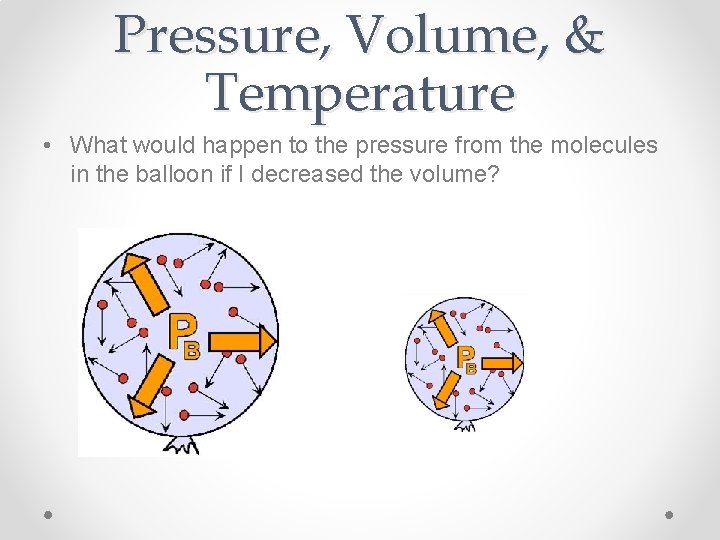
Pressure, Volume, & Temperature • What would happen to the pressure from the molecules in the balloon if I decreased the volume?

Pressure & Volume • Decreasing the Volume would Increase the Pressure V P • There is an inverse relationship between pressure and volume!

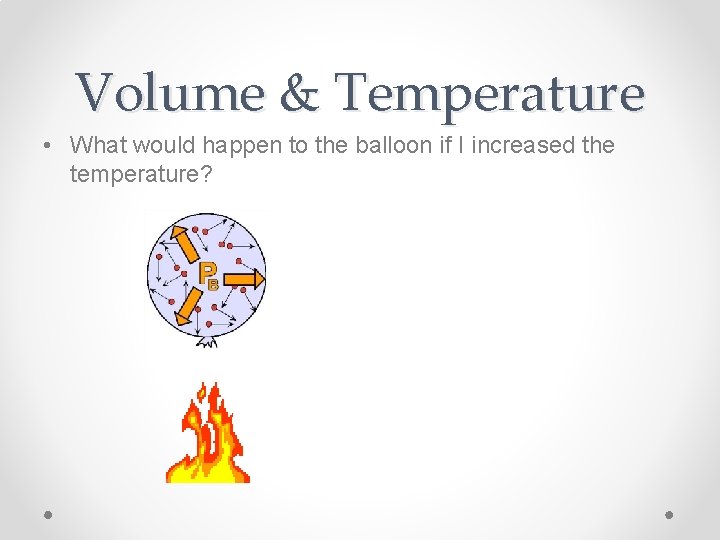
Volume & Temperature • What would happen to the balloon if I increased the temperature?

Volume & Temperature • Adding heat would increase the speed of the molecules, which increases the pressure inside the balloon, which increases the volume!

 Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Red liquid element
Red liquid element Molecular theory of gases and liquids
Molecular theory of gases and liquids The attraction between particles gives solids a definite
The attraction between particles gives solids a definite Thermal expansion and contraction examples
Thermal expansion and contraction examples Buoyancyability
Buoyancyability Molecules of solid liquid and gas
Molecules of solid liquid and gas States of matter diagram
States of matter diagram The properties of solids liquids and gases
The properties of solids liquids and gases Why is gas easier to compress than a liquid
Why is gas easier to compress than a liquid Solid liquid gas examples
Solid liquid gas examples Filter medium resistance formula
Filter medium resistance formula Liquids and solids menu
Liquids and solids menu Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers






























