The Islamic University of Gaza Environmental Engineering Department






- Slides: 17

The Islamic University of Gaza- Environmental Engineering Department Environmental Measurements (EENV 4244) Lecture 4: Free chlorine and hypochlorite Prepared by Husam Al-Najar



Introduction Why we should measure chlorine? What do we mean by residual chlorine? What is the source for presence of chlorine in water and wastewater? Is it mandatory to measure residual chlorine in water and wastewater? Treated of filtered water is deemed to be fit for consumption only if it is devoid of diseases producing microorganism. Chlorination is primarily adopted to destroy or deactivate diseases- producing microorganisms in the public water supply. Chlorine is added to water, some of the chlorine reacts first with organic materials and metals in the water and is not available for disinfection (this is called chlorine demand of the water). The remaining chlorine concentration after the chlorine demand is accounted for is called total chlorine.

Chlorine is one of the most widely used disinfectants. It is very applicable and very effective for the deactivation of pathogenic microorganisms. Chlorine can be easily applied, measures and controlled. It is relatively cheap. However, we only started using it as a disinfectants on a wider scale in the nineteenth century, after Louis Pasteur discovered that microorganisms spread certain diseases. Chlorine has played an important role in lengthening the life-expectancy of humans. Chlorine can be used as a disinfectant of the following forms: § Gaseous form Cl 2. Bleaching solution (Na. OH): Bleach consists of chlorine gas dissolved in an alkali -solution, such as sodium hydroxide (Na. OH). Chlorine reacts with sodium hydroxide to sodium hypochlorite (Na. OCl). § Bleaching powder Ca(OCl)2: This is produced by directing chlorine through calcium hydroxide (Ca. OH). The benefit of bleaching powder is that it is a solid. §

Water borne diseases

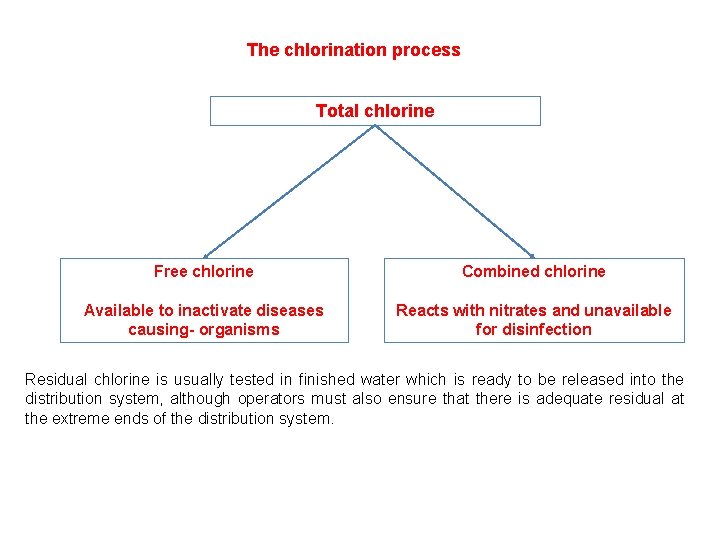
The chlorination process Total chlorine Free chlorine Combined chlorine Available to inactivate diseases causing- organisms Reacts with nitrates and unavailable for disinfection Residual chlorine is usually tested in finished water which is ready to be released into the distribution system, although operators must also ensure that there is adequate residual at the extreme ends of the distribution system.

• Chlorine readily combines with chemicals dissolved in water, microorganisms, small animals, plant material, tastes, odors, and colors. • These components "use up" chlorine and comprise the chlorine demand of the treatment system. • It is important to add sufficient chlorine to the water to meet the chlorine demand provide residual disinfection. 1. Chlorine demand is the amount of chlorine required to kill bacteria, oxidize iron or other elements in the water. 2. Free available chlorine residual is the amount of chlorine remaining in the water after the chlorine demand has been met. 3. Contact time is the amount of time that the chlorine is present in the water. The combination of chlorine residual and contact time determines the effectiveness of the chlorination treatment.

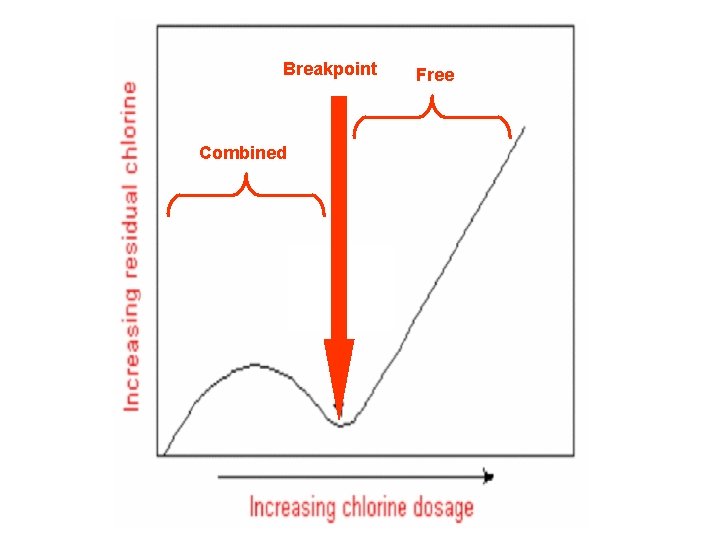
Breakpoint Combined Free

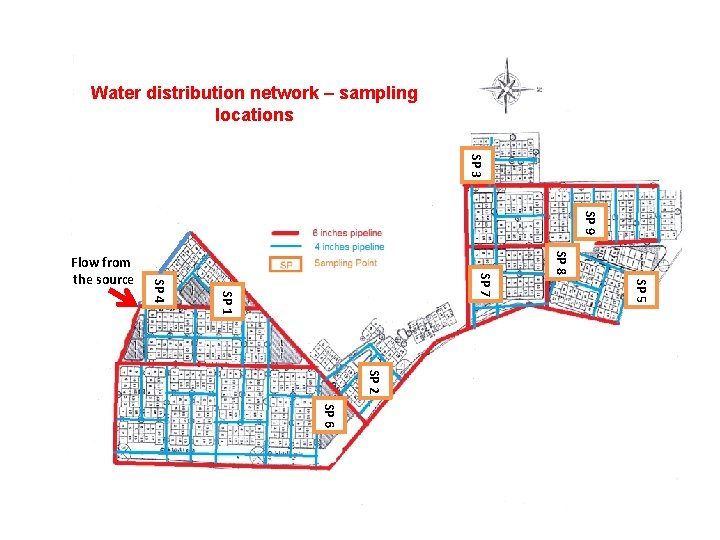
Water distribution network – sampling locations SP 3 SP 9 SP 8 SP 5 SP 7 SP 1 SP 4 Flow from the source SP 2 SP 6

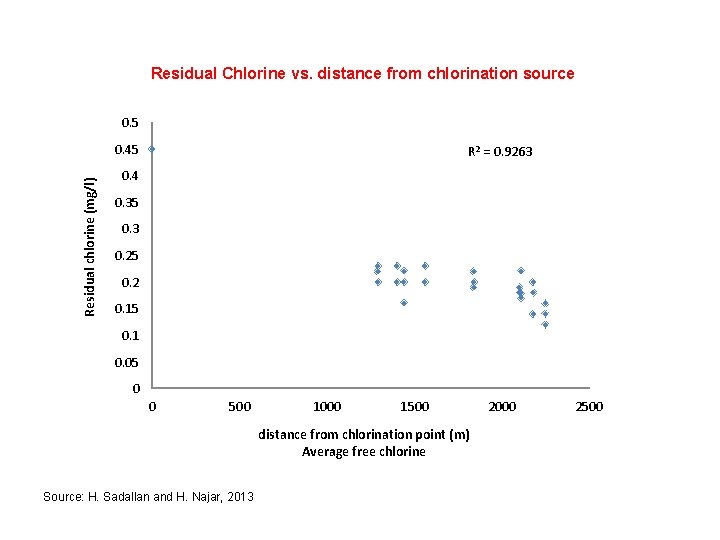
Residual Chlorine vs. distance from chlorination source 0. 5 Residual chlorine (mg/l) 0. 45 R 2 = 0. 9263 0. 4 0. 35 0. 3 0. 25 0. 2 0. 15 0. 1 0. 05 0 0 500 1000 1500 distance from chlorination point (m) Average free chlorine Source: H. Sadallan and H. Najar, 2013 2000 2500

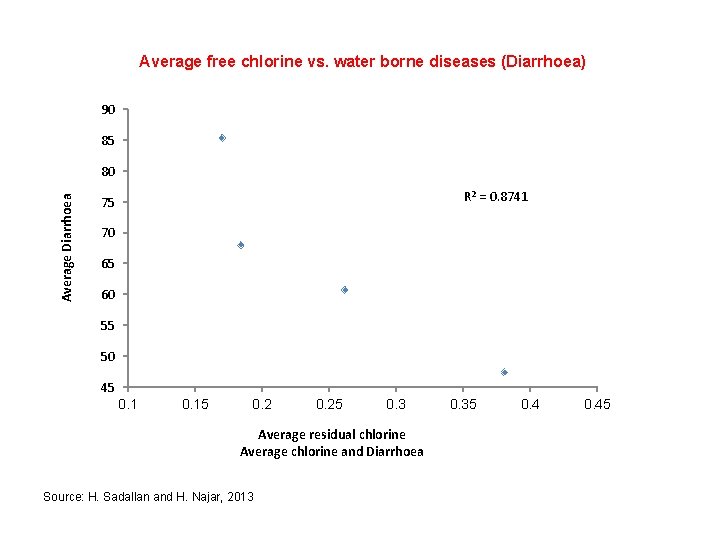
Average free chlorine vs. water borne diseases (Diarrhoea) 90 85 Average Diarrhoea 80 R 2 = 0. 8741 75 70 65 60 55 50 45 0. 15 0. 25 0. 3 Average residual chlorine Average chlorine and Diarrhoea Source: H. Sadallan and H. Najar, 2013 0. 35 0. 45

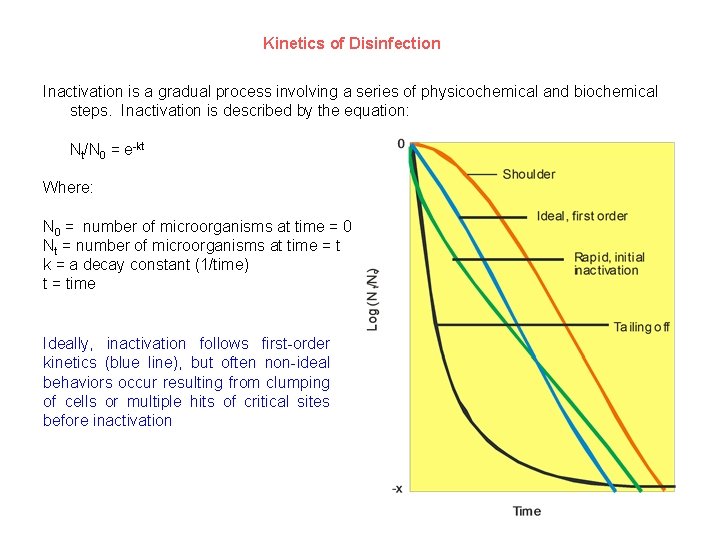
Kinetics of Disinfection Inactivation is a gradual process involving a series of physicochemical and biochemical steps. Inactivation is described by the equation: Nt/N 0 = e-kt Where: N 0 = number of microorganisms at time = 0 Nt = number of microorganisms at time = t k = a decay constant (1/time) t = time Ideally, inactivation follows first-order kinetics (blue line), but often non-ideal behaviors occur resulting from clumping of cells or multiple hits of critical sites before inactivation

Concentration and Contact Time Effectiveness of chlorination depends primarily on the concentration used and the time of exposure Disinfectant effectiveness can be expressed as a C ▪ t value where: C = disinfectant concentration t = time required to inactivate a 99% of the population under specific conditions The lower the C ▪ t, the more effective the disinfectant In general, resistance to disinfection is in the following order: vegetative bacteria < enteric viruses < spore-forming bacteria < protozoan cysts

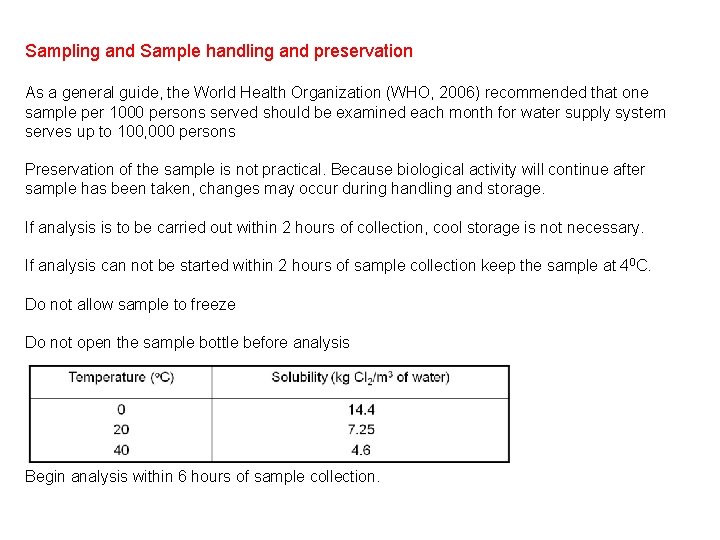
Sampling and Sample handling and preservation As a general guide, the World Health Organization (WHO, 2006) recommended that one sample per 1000 persons served should be examined each month for water supply system serves up to 100, 000 persons Preservation of the sample is not practical. Because biological activity will continue after sample has been taken, changes may occur during handling and storage. If analysis is to be carried out within 2 hours of collection, cool storage is not necessary. If analysis can not be started within 2 hours of sample collection keep the sample at 40 C. Do not allow sample to freeze Do not open the sample bottle before analysis Begin analysis within 6 hours of sample collection.

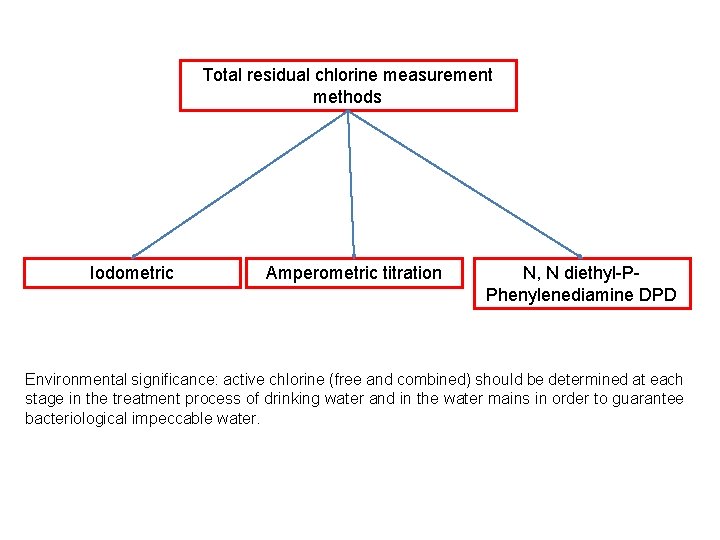
Total residual chlorine measurement methods Iodometric Amperometric titration N, N diethyl-PPhenylenediamine DPD Environmental significance: active chlorine (free and combined) should be determined at each stage in the treatment process of drinking water and in the water mains in order to guarantee bacteriological impeccable water.

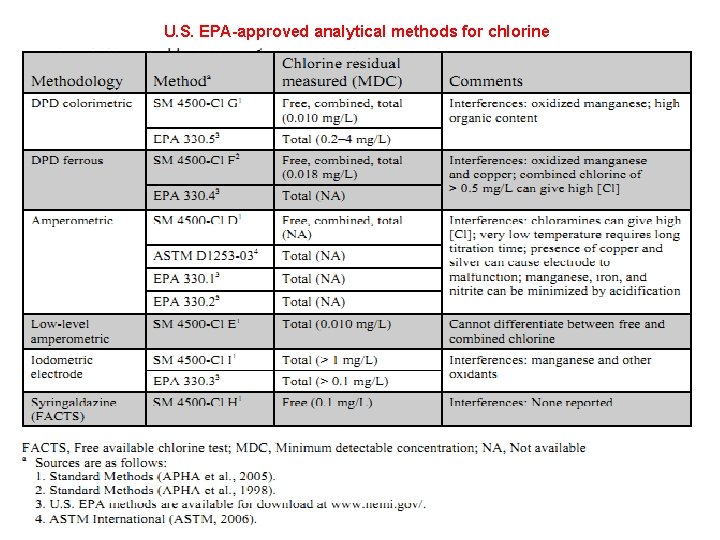
U. S. EPA-approved analytical methods for chlorine
 Sınıfı
Sınıfı Pakimires
Pakimires Bil soruyu bas gaza
Bil soruyu bas gaza Gaza strip map
Gaza strip map Gaza strip map
Gaza strip map Mapa franja de gaza
Mapa franja de gaza Estado de israel palestina
Estado de israel palestina Gaza central desalination plant
Gaza central desalination plant Gáza
Gáza Engineering temple run
Engineering temple run Department of information engineering university of padova
Department of information engineering university of padova Information engineering padova
Information engineering padova University of sargodha engineering department
University of sargodha engineering department Islamic online university malaysia
Islamic online university malaysia Islamic university college
Islamic university college Wireless health
Wireless health Nyc department of environmental protection
Nyc department of environmental protection Ladeq
Ladeq

































