The Interdependence of math and science The Interdependence

- Slides: 12

• The Interdependence of math and science The Interdependence of Math and Science: You really can't learn one without the other. • Unfortunately, science is often presented to high school students without the math that students need to truly understand the subject. • You see, Mathematics and Science are Inseparable. Math has no point unless it is describing aspects of nature or economics, and science cannot study nature without using the concept of scale and mathematical descriptions of natural phenomena. • Any attempt to take the math out of the natural sciences so that biology, chemistry and physics will be easier for students, is like taking the food away from lunch so that the meal is easier to swallow. It just doesn't make sense, and ultimately such a strategy cheats students out of their education. What does interdependent mean? Why are math and science interdependent? Slide by: J. Levasseur ©

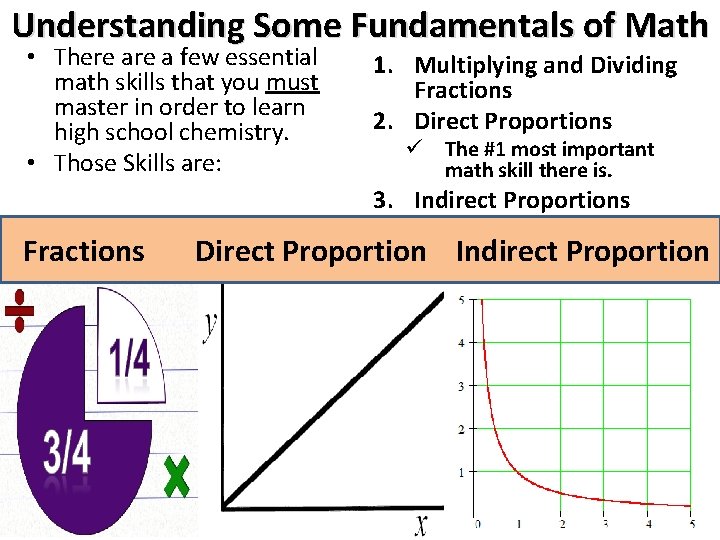
Understanding Some Fundamentals of Math • There a few essential math skills that you must master in order to learn high school chemistry. • Those Skills are: 1. Multiplying and Dividing Fractions 2. Direct Proportions The #1 most important math skill there is. ü 3. Indirect Proportions Fractions Direct Proportion Indirect Proportion 5 T h i s This 4 3 2 1 This per That 0 1 2 That 3 4 5

A Very Significant Problem: How many students per room? • There are in fact 2200 students at Springfield Central High School. • There are 88 classrooms at Central. • If the students are evenly disbursed to all the classrooms, what is the number of students per room? There will be 25 students PER room Here it is; if you can solve this problem you can solve just about any chemistry problem you will ever see in this building

Question: How Do We Use the Concept of Scale to Understand Matter’s Changes? Measurements Comparing matter and energy against clear standards of measurements, measurements we can communicate more effectively in answering simple questions. Mass (measured in Grams or Kilograms) How heavy is an egg? Volume (measured in m 3, cm 3 or Liters) How much space does an egg occupy? Density (measured in kg/m 3 , g/cm 3) Does an egg size piece of steel or wood have the same mass? Pressure (measured in torr, atm, mm Hg) How hard is it to crush an egg by squeezing evenly from all sides? Amount (measured in dozens, or in chemistry Moles) How many eggs come in a standard carton of eggs? Temperature (measure in ˚Fahrenheit, ˚Celsius or Kelvin) At what temperature will an egg cook?

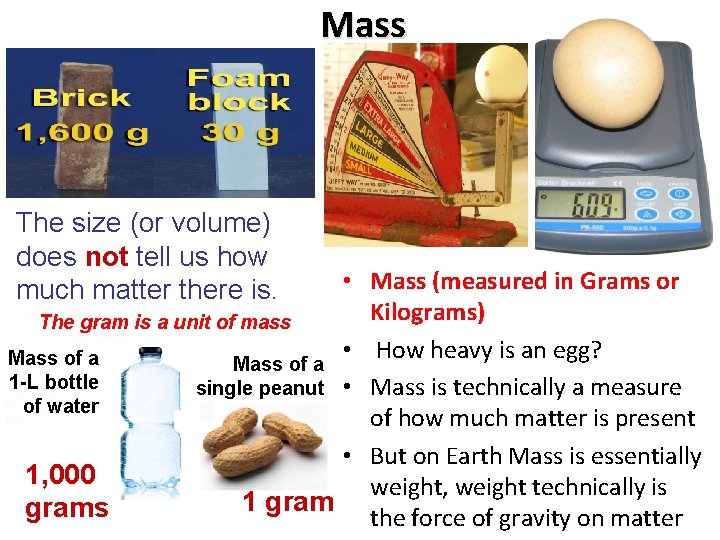
Mass The size (or volume) does not tell us how much matter there is. • Mass (measured in Grams or Kilograms) The gram is a unit of mass • How heavy is an egg? Mass of a 1 -L bottle single peanut • Mass is technically a measure of water of how much matter is present • But on Earth Mass is essentially 1, 000 weight, weight technically is 1 grams the force of gravity on matter

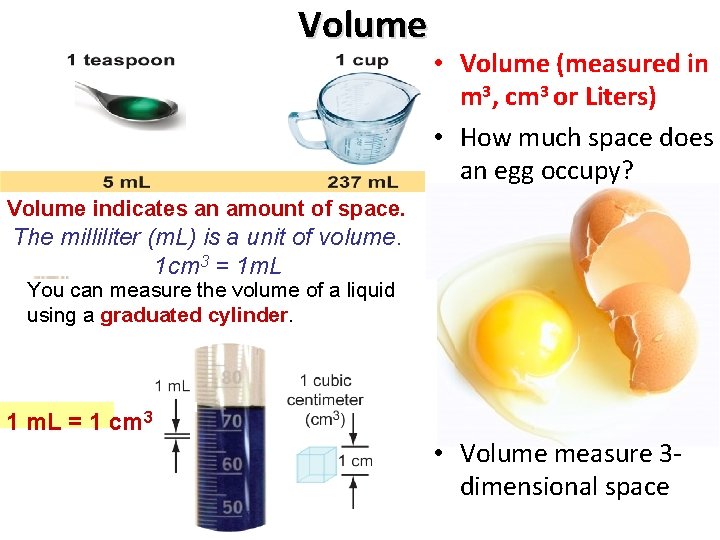
Volume • Volume (measured in m 3, cm 3 or Liters) • How much space does an egg occupy? Volume indicates an amount of space. The milliliter (m. L) is a unit of volume. 1 cm 3 = 1 m. L You can the volume of a liquid Youmeasure can measure the volume of a using liquid a graduated using acylinder. graduated cylinder. 1 m. L = 1 cm 3 75 m. L • Volume measure 3 dimensional space

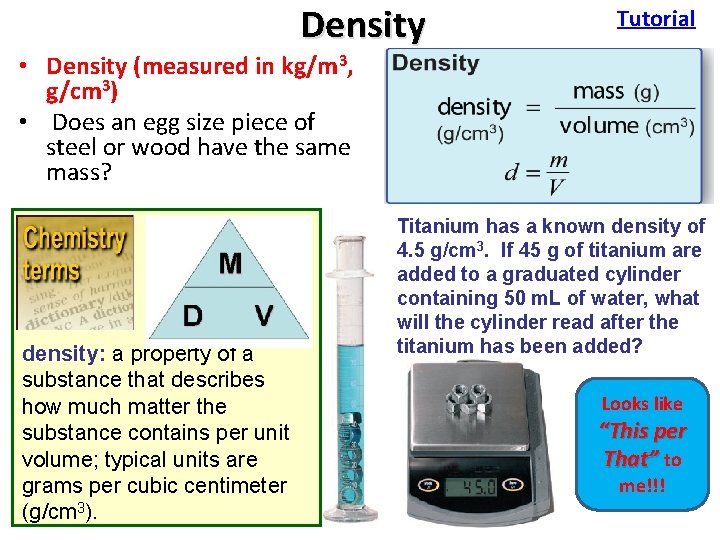
Density Tutorial • Density (measured in kg/m 3, g/cm 3) • Does an egg size piece of steel or wood have the same mass? density: a property of a substance that describes how much matter the substance contains per unit volume; typical units are grams per cubic centimeter (g/cm 3). Titanium hasof a known density of 3 blocks equal volume Which of these three cube has theare 3. If 45 4. 5 g/cm g of titanium most volume? added to a graduated cylinder Which of these cubes has what the containing 50 three m. L of water, glass read afteriron most mass? plastic will the cylinder the What is thehas density ofadded? each cube? titanium been Looks like “This per That” to 1 cm 3 me!!! 1 cm 3 3 different mass values

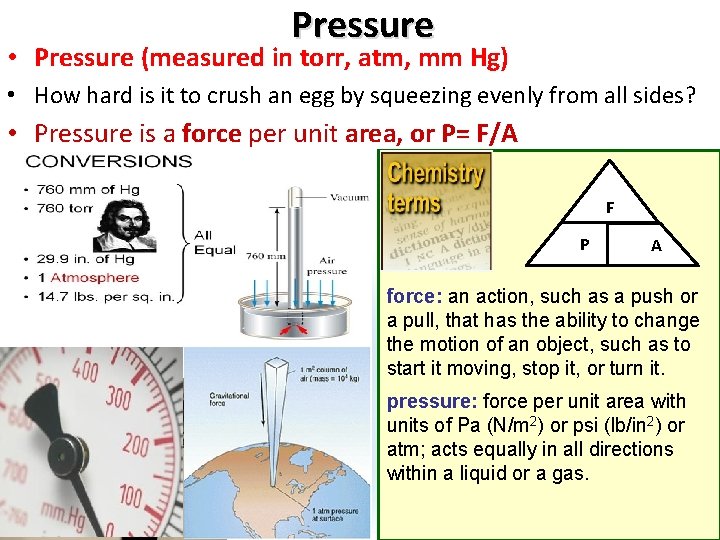
Pressure • Pressure (measured in torr, atm, mm Hg) • How hard is it to crush an egg by squeezing evenly from all sides? • Pressure is a force per unit area, or P= F/A F P A force: an action, such as a push or a pull, that has the ability to change the motion of an object, such as to start it moving, stop it, or turn it. pressure: force per unit area with units of Pa (N/m 2) or psi (lb/in 2) or atm; acts equally in all directions within a liquid or a gas.

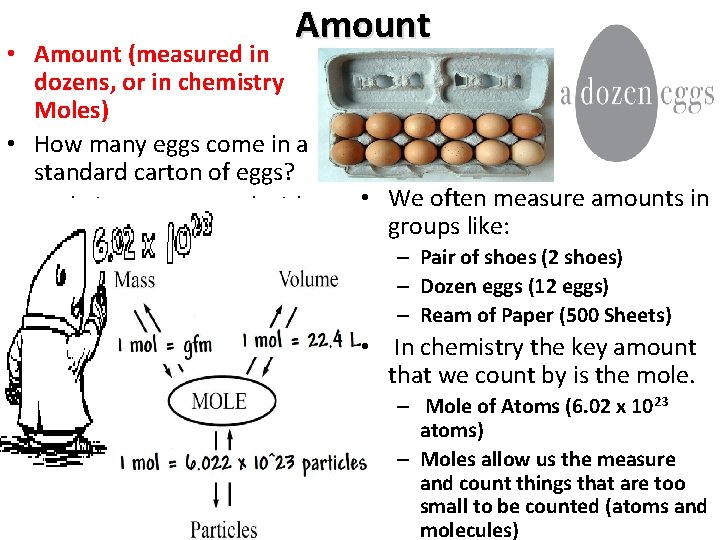
Amount • Amount (measured in dozens, or in chemistry Moles) • How many eggs come in a standard carton of eggs? • Mole is a count word with a value of 6. 02 x 1023 • We often measure amounts in groups like: – Pair of shoes (2 shoes) – Dozen eggs (12 eggs) – Ream of Paper (500 Sheets) • In chemistry the key amount that we count by is the mole. – Mole of Atoms (6. 02 x 1023 atoms) – Moles allow us the measure and count things that are too small to be counted (atoms and molecules)

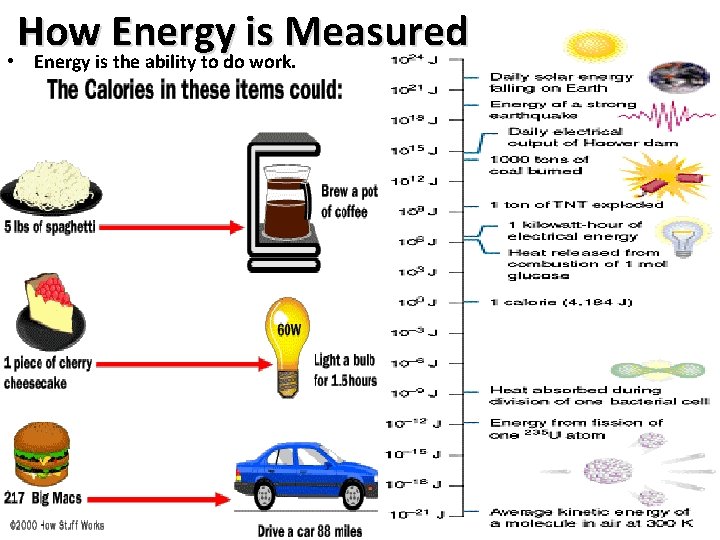
How Energy is Measured • Energy is the ability to do work. – Work is the ability to move objects. • Energy is measured in units called joules. • A joule is the SI (international measuring system) unit of electrical, mechanical, and thermal energy – A joule is a unit of electrical energy equal to the work done when a current of one ampere is passed through a resistance of ØMatter and energy are closely associated: one ohm for one second. ØE=mc 2 says matter is energy, – A joule is likewise a unit of energy equal (but you need a nuclear reaction to the work done when a force of one Newton acts through a distance of one to get that energy) meter. ØChemical compounds also hold – 1 calorie = 4. 184 joules energy in the chemical bonds; you break those bonds in a chemical • Calorie is a common unit of measuring energy reaction to release the energy. ØThis is how fuel and food • A calorie is the energy, (heat) required to raise 1 gram of water 1 give energy. degree Celsius http: //www. answers. com/topic/joule Prentice Hall Chem. Guide Essentials of Chemistry 2002

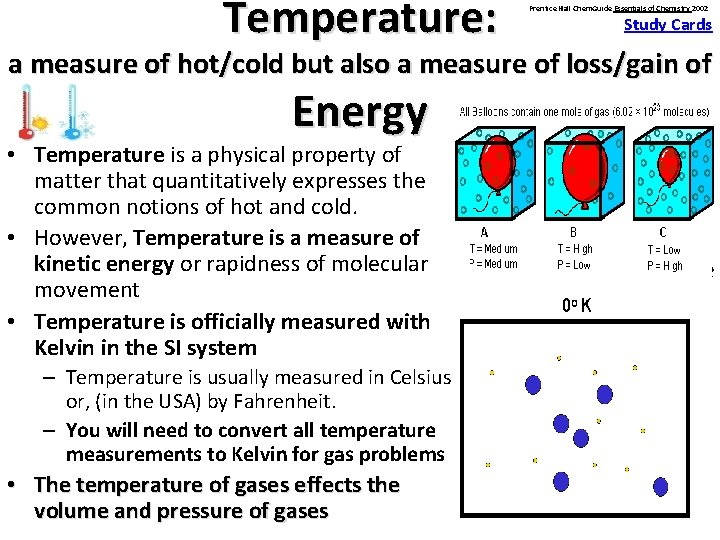
Temperature: Prentice Hall Chem. Guide Essentials of Chemistry 2002 Study Cards a measure of hot/cold but also a measure of loss/gain of Energy § Temperature • Temperature is a physical property of (measure in matter that quantitatively expresses the ˚Fahrenheit, ˚Celsius common notions of hot and cold. or Kelvin) • However, Temperature is a measure of kinetic energy or rapidness of molecular § At what temperature movement will an egg cook? • Temperature is officially measured with Kelvin in the SI system – Temperature is usually measured in Celsius or, (in the USA) by Fahrenheit. – You will need to convert all temperature measurements to Kelvin for gas problems • The temperature of gases effects the volume and pressure of gases

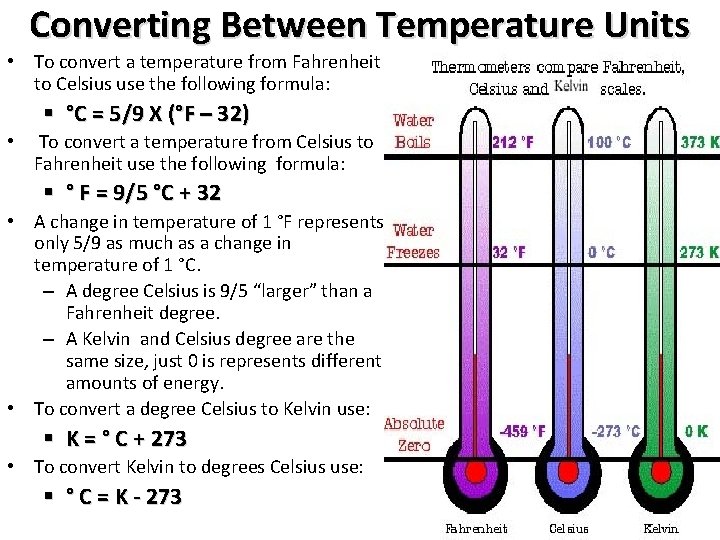
Converting Between Temperature Units • To convert a temperature from Fahrenheit to Celsius use the following formula: § °C = 5/9 X (°F – 32) • To convert a temperature from Celsius to Fahrenheit use the following formula: § ° F = 9/5 °C + 32 • A change in temperature of 1 °F represents only 5/9 as much as a change in temperature of 1 °C. – A degree Celsius is 9/5 “larger” than a Fahrenheit degree. – A Kelvin and Celsius degree are the same size, just 0 is represents different amounts of energy. • To convert a degree Celsius to Kelvin use: § K = ° C + 273 • To convert Kelvin to degrees Celsius use: To Solve Gas Law Problems You need to know how to convert TO Kelvin: • If given Celsius: • Add 273 • K = ° C + 273 • If given Fahrenheit: • Convert to Celsius then add 273 • °C = 5/9 X (°F – 32) • K = ° C + 273 § ° C = K - 273 Prentice Hall Chem. Guide Essentials of Chemistry 2002