The initial oxidation step of saturated aliphatic hydrocarbons
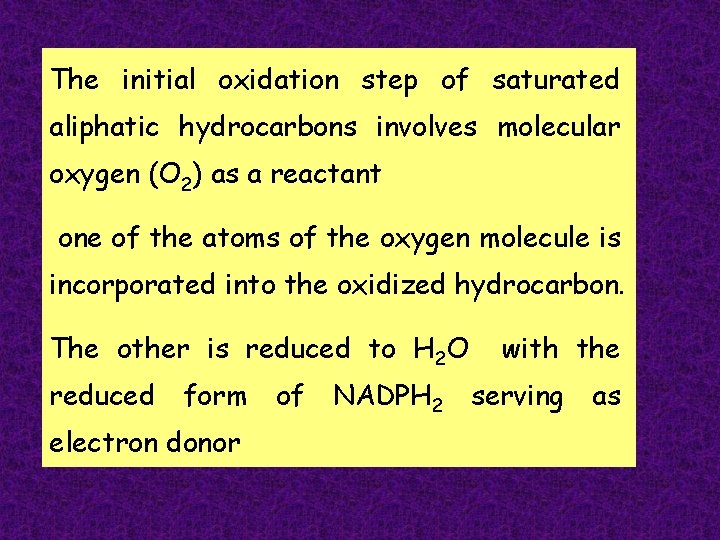
The initial oxidation step of saturated aliphatic hydrocarbons involves molecular oxygen (O 2) as a reactant one of the atoms of the oxygen molecule is incorporated into the oxidized hydrocarbon. The other is reduced to H 2 O reduced form electron donor of NADPH 2 with the serving as
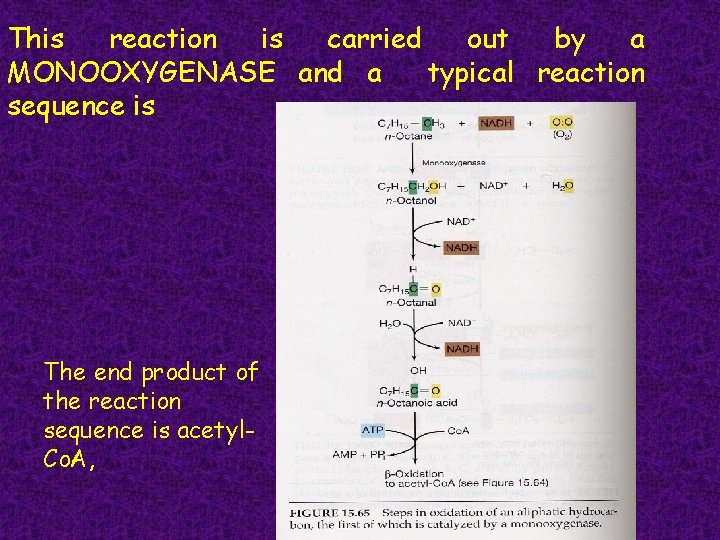
This reaction is carried out by a MONOOXYGENASE and a typical reaction sequence is The end product of the reaction sequence is acetyl. Co. A,
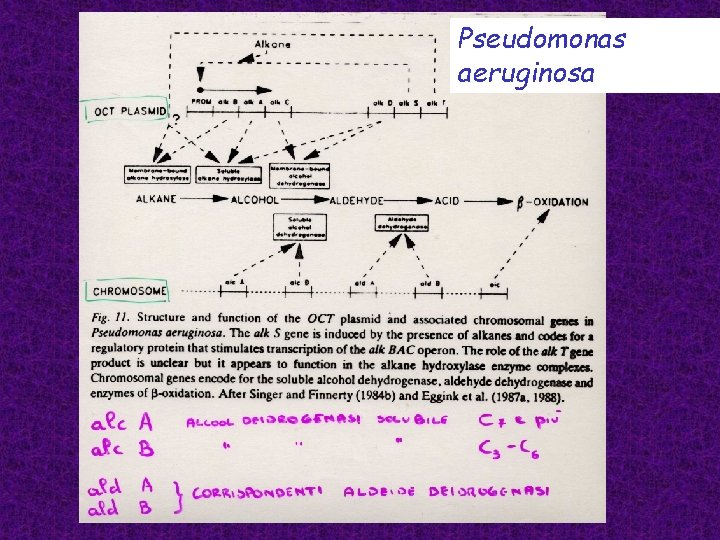
Pseudomonas aeruginosa


Di-terminal oxidation or w oxidation
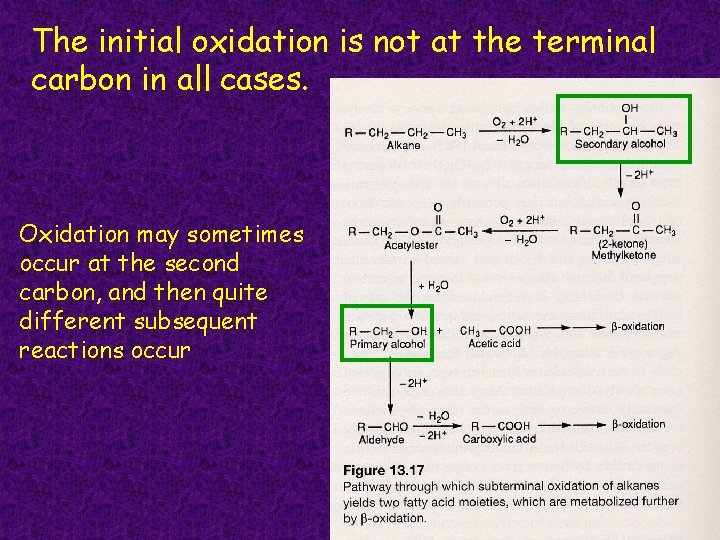
The initial oxidation is not at the terminal carbon in all cases. Oxidation may sometimes occur at the second carbon, and then quite different subsequent reactions occur
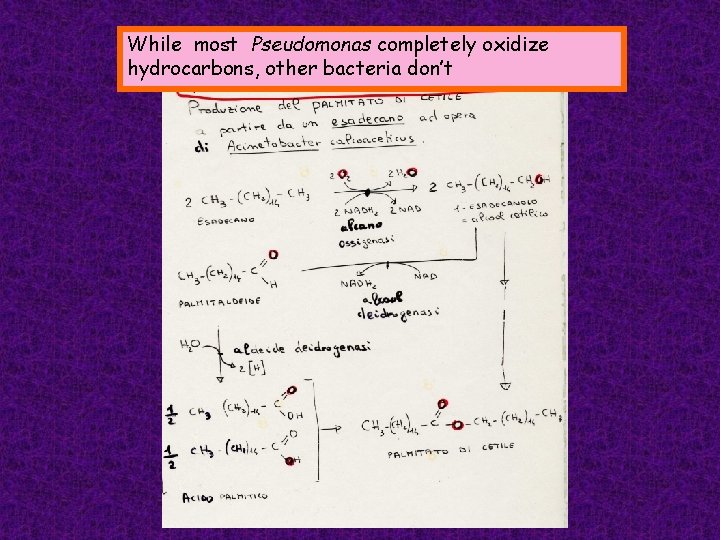
While most Pseudomonas completely oxidize hydrocarbons, other bacteria don’t

Unsaturated aliphatic hydrocarbons containing a terminal double bond are most likely degraded in anaerobic conditions and can be oxidized by certain sulfatereducing and other anaerobic bacteria
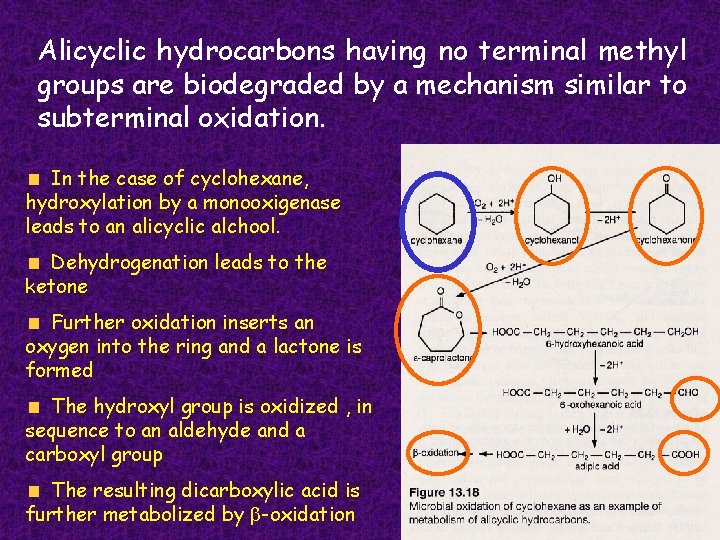
Alicyclic hydrocarbons having no terminal methyl groups are biodegraded by a mechanism similar to subterminal oxidation. In the case of cyclohexane, hydroxylation by a monooxigenase leads to an alicyclic alchool. Dehydrogenation leads to the ketone Further oxidation inserts an oxygen into the ring and a lactone is formed The hydroxyl group is oxidized , in sequence to an aldehyde and a carboxyl group The resulting dicarboxylic acid is further metabolized by b-oxidation
Many aromatic hydrocarbons can be used as electron donors and carbon source aerobically by bacteria of the genus Pseudomonas It has been demonstrated that the metabolism of these compounds, some of which are quite large molecules, frequently has as its initial stage the formation of protocatechuate or catechol, or a structurally related either compound
These single-ring compounds are referred to as starting substrates because oxidative catabolism proceeds only after the large aromatic molecules have been converted to these more simple forms. Protocatechuate and catechol may then be further degraded to compounds that can enter the citric acid cycle: succinate, acetyl-Co. A, pyruvate. Several steps in the catabolism of aromatic hydrocarbons usually require oxygenases (monooxygenases or dioxygenases).
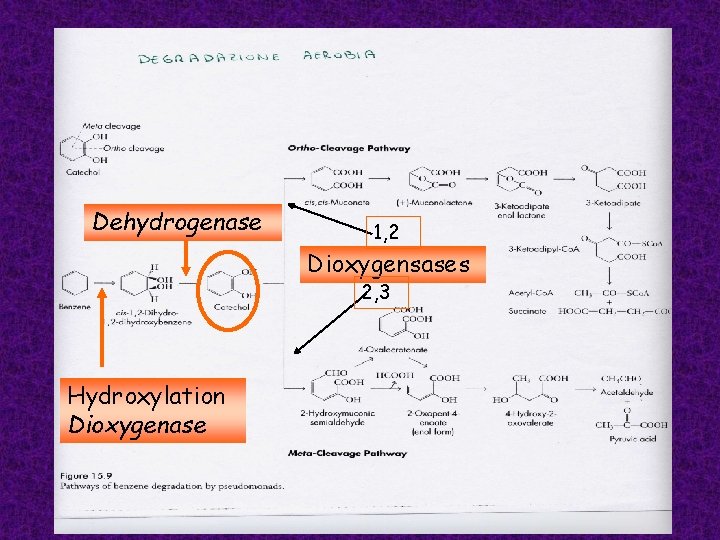
Dehydrogenase 1, 2 Dioxygensases 2, 3 Hydroxylation Dioxygenase
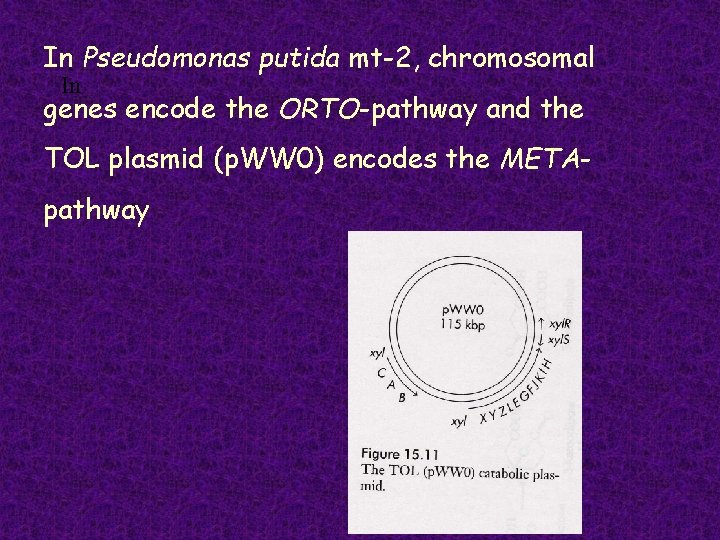
In Pseudomonas putida mt-2, chromosomal In genes encode the ORTO-pathway and the TOL plasmid (p. WW 0) encodes the METApathway
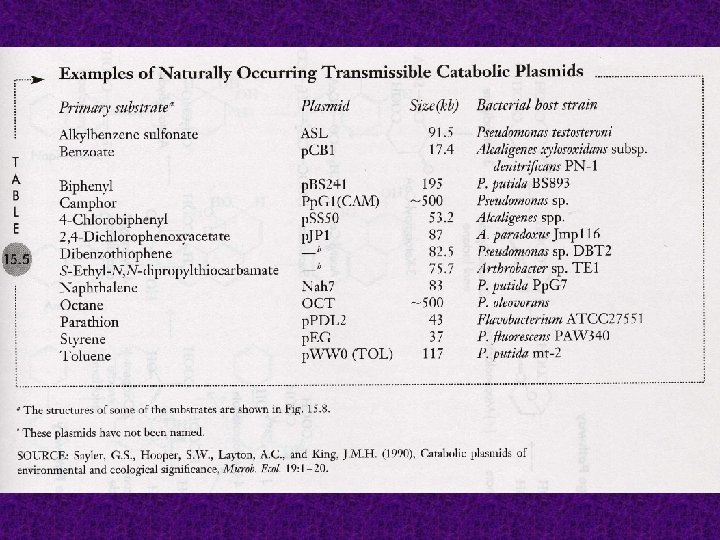

Aromatic compounds can also be degraded anaerobically and quite readily if they already contain an atom of oxygen. Anoxic mixed cultures degrade have been BENZOATE shown and to other substituted phenolic compounds yielding CH 4 and CO 2 as final products

SUBSTITUTED PHENOLIC COMPOUNDS are also degraded by certain denitrifying, phototrophic, ferric iron-reducing, and sulfatereducing bacteria The anoxic catabolism of aromatic compounds proceeds by reductive rather than oxidative ring cleavage
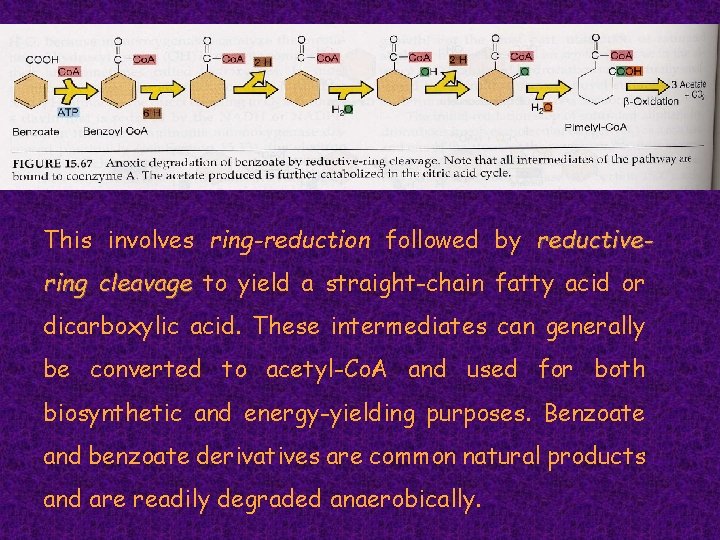
This involves ring-reduction followed by reductivering cleavage to yield a straight-chain fatty acid or dicarboxylic acid. These intermediates can generally be converted to acetyl-Co. A and used for both biosynthetic and energy-yielding purposes. Benzoate and benzoate derivatives are common natural products and are readily degraded anaerobically.

Evidence for the anoxic degradation of benzene and toluene, aromatic compounds lacking an oxygen atom has also been obtained. Catabolism of benzene occurs by anaerobic microbial consortia leading to methanogenesis, but toluene oxidation to CO 2 can occur in pure culture
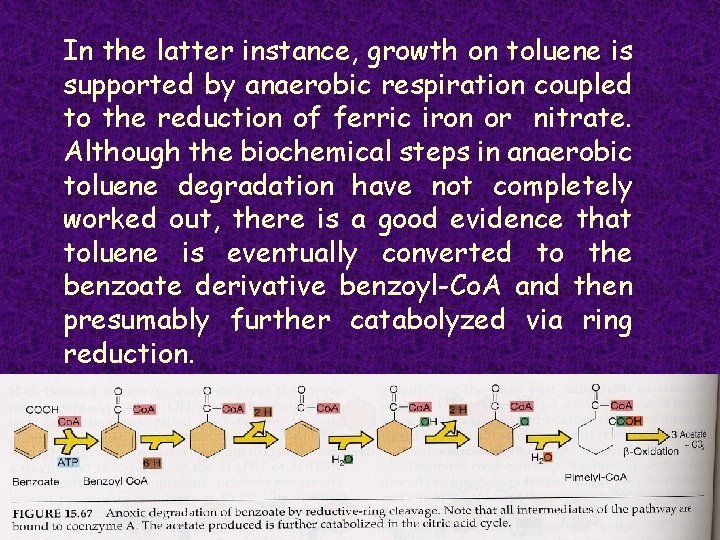
In the latter instance, growth on toluene is supported by anaerobic respiration coupled to the reduction of ferric iron or nitrate. Although the biochemical steps in anaerobic toluene degradation have not completely worked out, there is a good evidence that toluene is eventually converted to the benzoate derivative benzoyl-Co. A and then presumably further catabolyzed via ring reduction.
General rules for degradation of aliphatic compounds: A. Midsize straight-chain aliphatics (n-alkanes C 10 to C 18 in length) are utilized more readily than n-alkanes with either shorter or longer chains B. Saturated aliphatics degraded similarly and alkenes are C. Hydrocarbon branching biodegradability decreases D. Halogen substitution biodegradability decreases
- Slides: 20