The influence of different fixatives and preparation methods
- Slides: 1

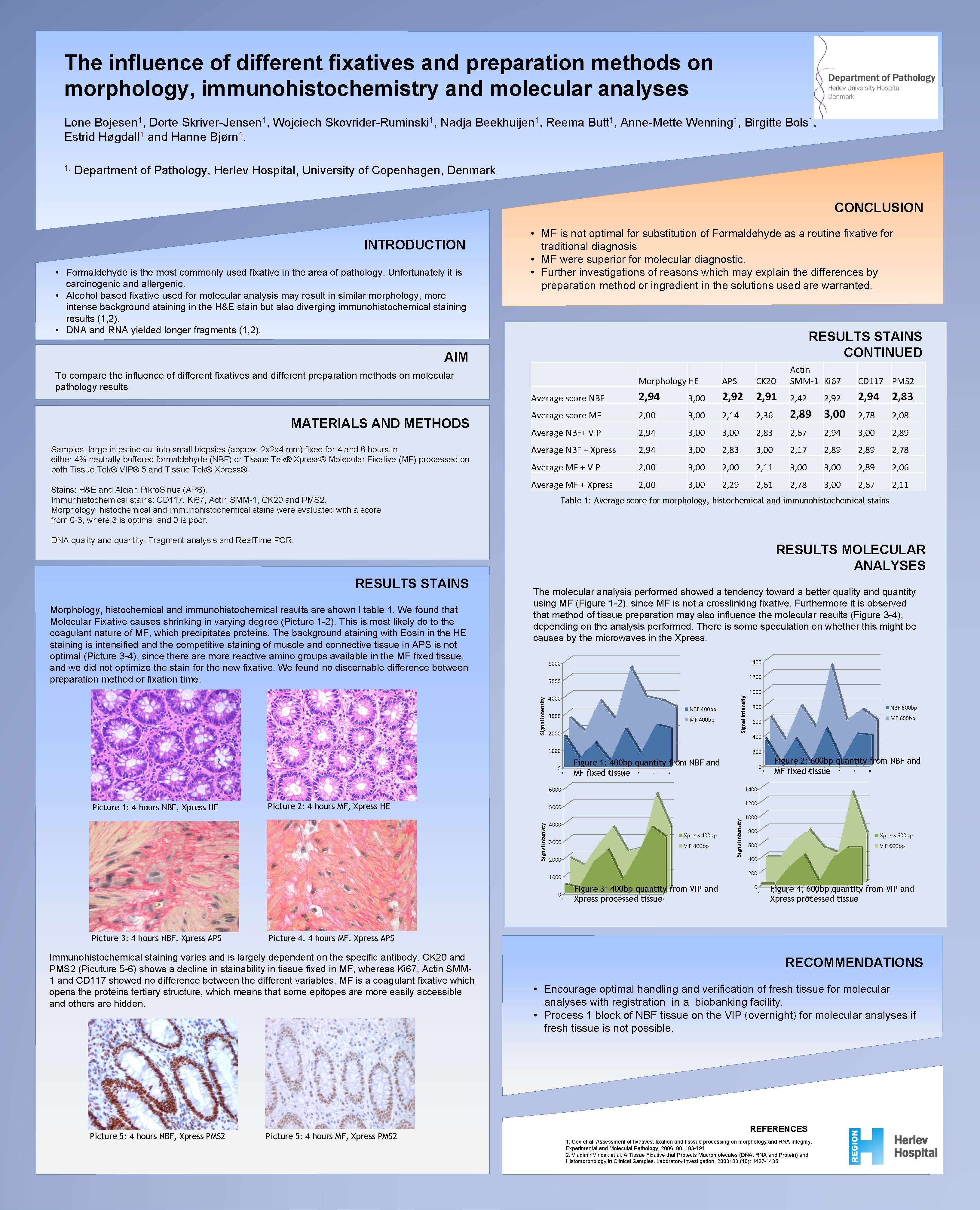
The influence of different fixatives and preparation methods on morphology, immunohistochemistry and molecular analyses Lone Bojesen 1, Dorte Skriver-Jensen 1, Wojciech Skovrider-Ruminski 1, Nadja Beekhuijen 1, Reema Butt 1, Anne-Mette Wenning 1, Birgitte Bols 1, Estrid Høgdall 1 and Hanne Bjørn 1. 1. Department of Pathology, Herlev Hospital, University of Copenhagen, Denmark CONCLUSION INTRODUCTION • Formaldehyde is the most commonly used fixative in the area of pathology. Unfortunately it is carcinogenic and allergenic. • Alcohol based fixative used for molecular analysis may result in similar morphology, more intense background staining in the H&E stain but also diverging immunohistochemical staining results (1, 2). • DNA and RNA yielded longer fragments (1, 2). • MF is not optimal for substitution of Formaldehyde as a routine fixative for traditional diagnosis • MF were superior for molecular diagnostic. • Further investigations of reasons which may explain the differences by preparation method or ingredient in the solutions used are warranted. RESULTS STAINS CONTINUED AIM To compare the influence of different fixatives and different preparation methods on molecular pathology results MATERIALS AND METHODS Samples: large intestine cut into small biopsies (approx. 2 x 2 x 4 mm) fixed for 4 and 6 hours in either 4% neutrally buffered formaldehyde (NBF) or Tissue Tek® Xpress® Molecular Fixative (MF) processed on both Tissue Tek® VIP® 5 and Tissue Tek® Xpress®. Stains: H&E and Alcian Pikro. Sirius (APS). Immunhistochemical stains: CD 117, Ki 67, Actin SMM-1, CK 20 and PMS 2. Morphology, histochemical and immunohistochemical stains were evaluated with a score from 0 -3, where 3 is optimal and 0 is poor. Morphology HE APS CK 20 Actin SMM-1 Ki 67 Average score NBF 2, 94 3, 00 2, 92 2, 91 2, 42 2, 94 2, 83 Average score MF 2, 00 3, 00 2, 14 2, 36 2, 89 3, 00 2, 78 2, 08 Average NBF+ VIP 2, 94 3, 00 2, 83 2, 67 2, 94 3, 00 2, 89 Average NBF + Xpress 2, 94 3, 00 2, 83 3, 00 2, 17 2, 89 2, 78 Average MF + VIP 2, 00 3, 00 2, 11 3, 00 2, 89 2, 06 Average MF + Xpress 2, 00 3, 00 2, 29 2, 61 2, 78 3, 00 2, 67 2, 11 Table 1: Average score for morphology, histochemical and immunohistochemical stains DNA quality and quantity: Fragment analysis and Real. Time PCR. RESULTS MOLECULAR ANALYSES 1400 6000 1200 5000 Signal intensity Morphology, histochemical and immunohistochemical results are shown I table 1. We found that Molecular Fixative causes shrinking in varying degree (Picture 1 -2). This is most likely do to the coagulant nature of MF, which precipitates proteins. The background staining with Eosin in the HE staining is intensified and the competitive staining of muscle and connective tissue in APS is not optimal (Picture 3 -4), since there are more reactive amino groups available in the MF fixed tissue, and we did not optimize the stain for the new fixative. We found no discernable difference between preparation method or fixation time. The molecular analysis performed showed a tendency toward a better quality and quantity using MF (Figure 1 -2), since MF is not a crosslinking fixative. Furthermore it is observed that method of tissue preparation may also influence the molecular results (Figure 3 -4), depending on the analysis performed. There is some speculation on whether this might be causes by the microwaves in the Xpress. 4000 NBF 400 bp 3000 MF 400 bp 2000 Signal intensity RESULTS STAINS 1000 0 Picture 2: 4 hours MF, Xpress HE 1 2 3 4 5 6 7 400 0 Figure 2: 600 bp quantity from NBF and MF fixed tissue 1 2 3 4 5 6 7 8 1400 1200 4000 Xpress 400 bp 3000 VIP 400 bp 2000 Signal intensity MF 600 bp 1000 800 Xpress 600 bp 600 VIP 600 bp 400 200 1 Figure 3: 400 bp quantity from VIP and Xpress processed tissue 2 3 4 5 6 7 8 0 1 Figure 4: 600 bp quantity from VIP and Xpress processed tissue 2 3 4 5 6 7 8 Prøver Picture 4: 4 hours MF, Xpress APS Immunohistochemical staining varies and is largely dependent on the specific antibody. CK 20 and PMS 2 (Picuture 5 -6) shows a decline in stainability in tissue fixed in MF, whereas Ki 67, Actin SMM 1 and CD 117 showed no difference between the different variables. MF is a coagulant fixative which opens the proteins tertiary structure, which means that some epitopes are more easily accessible and others are hidden. Picture 5: 4 hours NBF, Xpress PMS 2 NBF 600 bp 600 8 1000 Picture 3: 4 hours NBF, Xpress APS 800 Figure 1: 400 bp quantity from NBF and MF fixed tissue 5000 0 1000 200 6000 Picture 1: 4 hours NBF, Xpress HE CD 117 PMS 2 Picture 5: 4 hours MF, Xpress PMS 2 RECOMMENDATIONS • Encourage optimal handling and verification of fresh tissue for molecular analyses with registration in a biobanking facility. • Process 1 block of NBF tissue on the VIP (overnight) for molecular analyses if fresh tissue is not possible. REFERENCES 1: Cox et al: Assessment of fixatives, fixation and tisssue processing on morphology and RNA integrity. Experimental and Moleculat Pathology. 2006; 80: 183 -191 2: Vladimir Vincek et al: A Tissue Fixative that Protects Macromolecules (DNA, RNA and Protein) and Histomorphology in Clinical Samples. Laboratory Investigation. 2003; 83 (10): 1427 -1435