The inconvenient truth IR spectroscopy Yongsik Lee 2008
















- Slides: 55

The inconvenient truth: IR spectroscopy Yongsik Lee 2008. 10

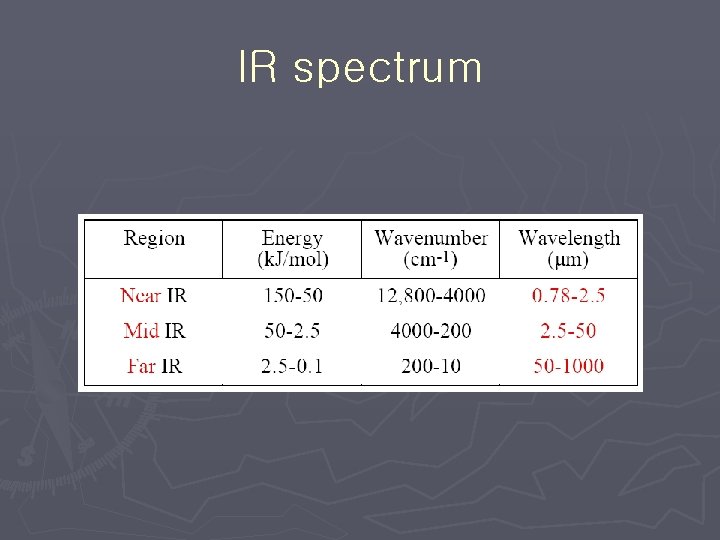
IR spectrum

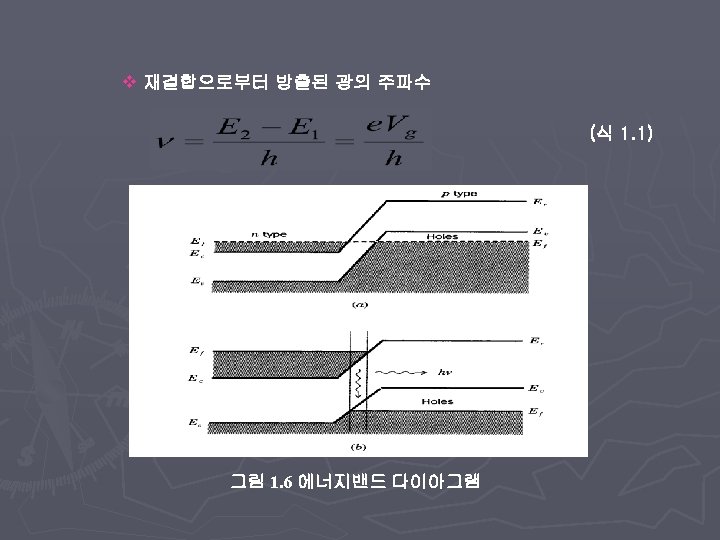
theory of IR ABSorption ► Energy of IR photon § insufficient to cause electronic excitation § But can cause vibrational or rotational excitation ► Fundamentals § Molecular electric field (dipole moment) interacts with IR photon electric field (both dynamic) ► Magnitude of dipole moment determined by § charge § separation of charge ► Vibration or rotation causes varying separation

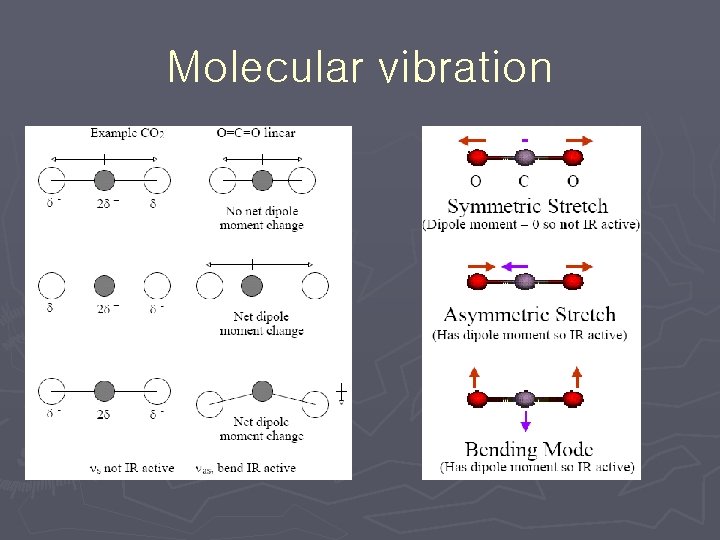
Dipole moment change ► Molecule must have change in dipole moment due to vibration or rotation to absorb IR radiation ► Absorption causes increase in vibration amplitude/rotation frequency ► Molecules with permanent dipole moments (µ) are IR active § HCl, CO, § H 2, N 2, § CO 2

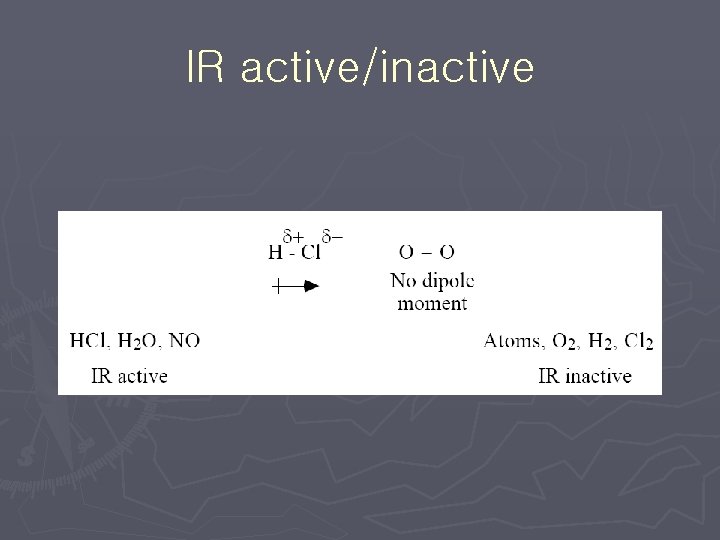
IR active/inactive

Electric Dipole moment

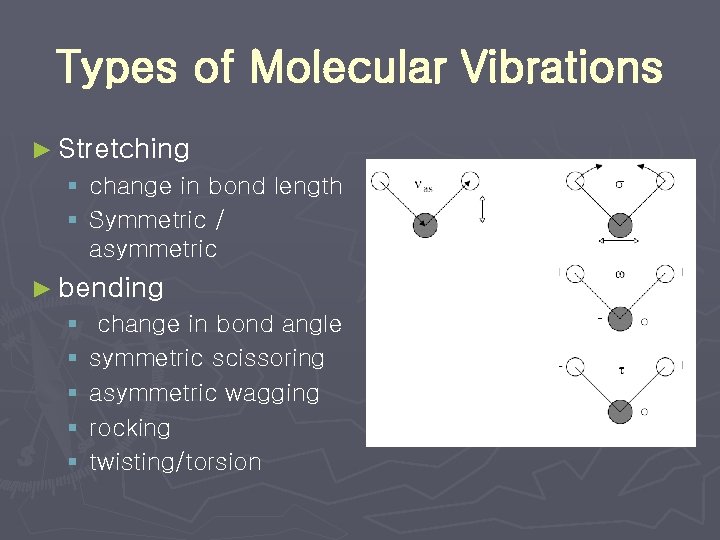
Types of Molecular Vibrations ► Stretching § change in bond length § Symmetric / asymmetric ► bending § § § change in bond angle symmetric scissoring asymmetric wagging rocking twisting/torsion

Molecular vibration

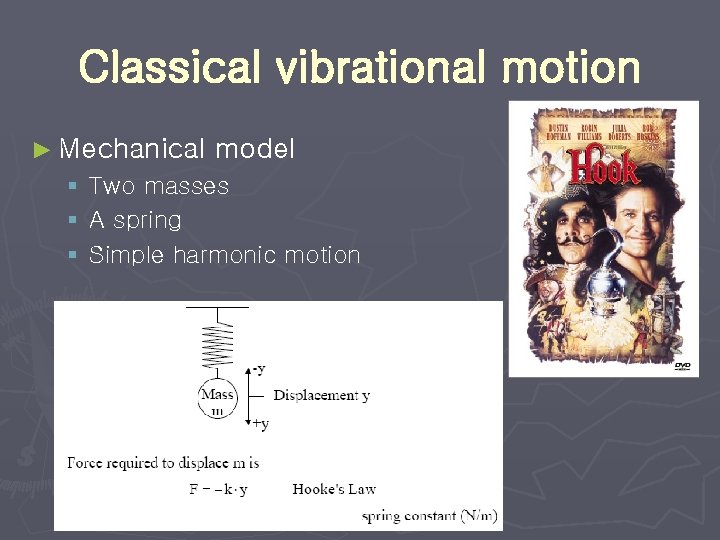
Classical vibrational motion ► Mechanical model § Two masses § A spring § Simple harmonic motion

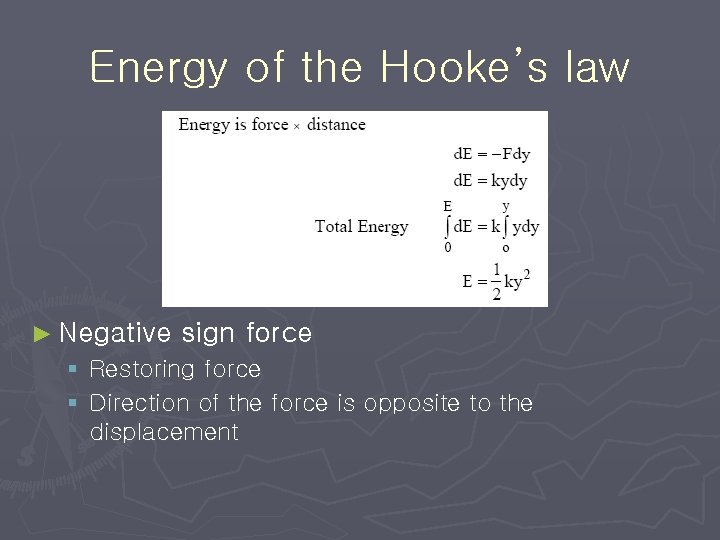
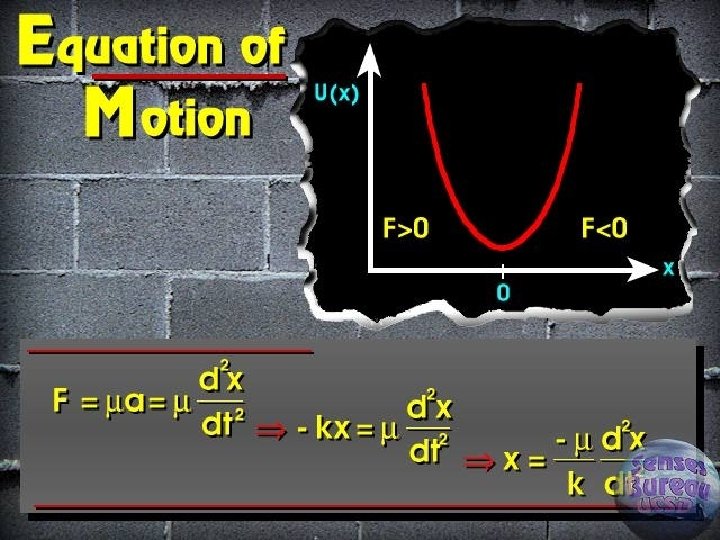
Energy of the Hooke’s law ► Negative sign force § Restoring force § Direction of the force is opposite to the displacement


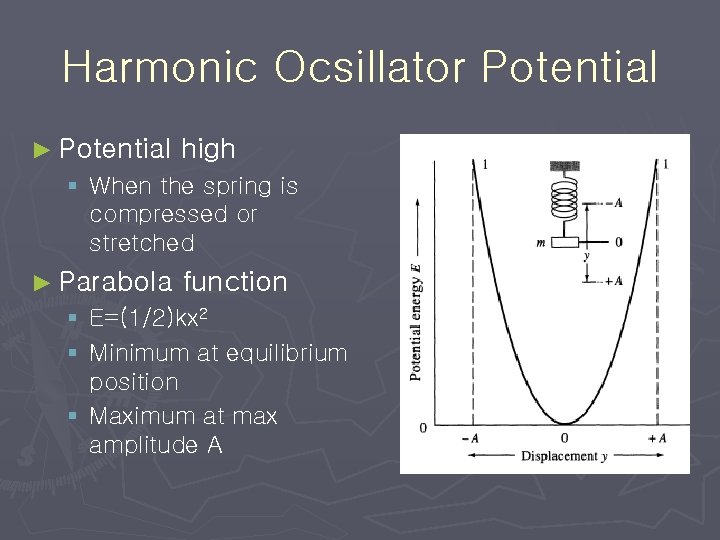
Harmonic Ocsillator Potential ► Potential high § When the spring is compressed or stretched ► Parabola function § E=(1/2)kx 2 § Minimum at equilibrium position § Maximum at max amplitude A

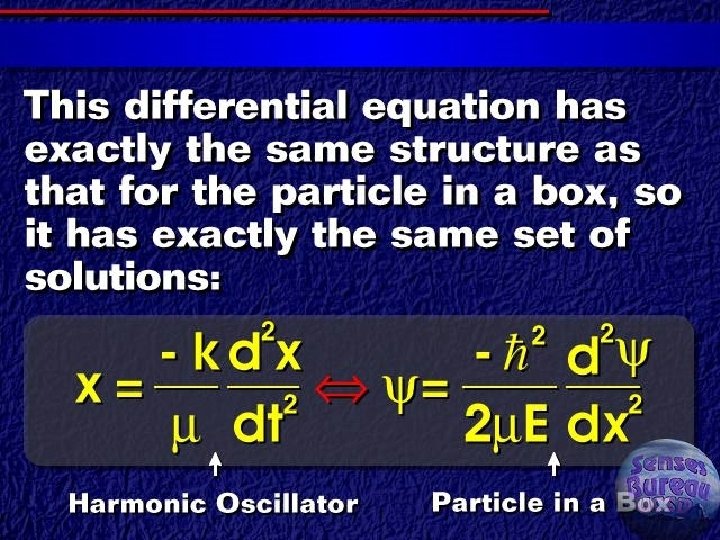
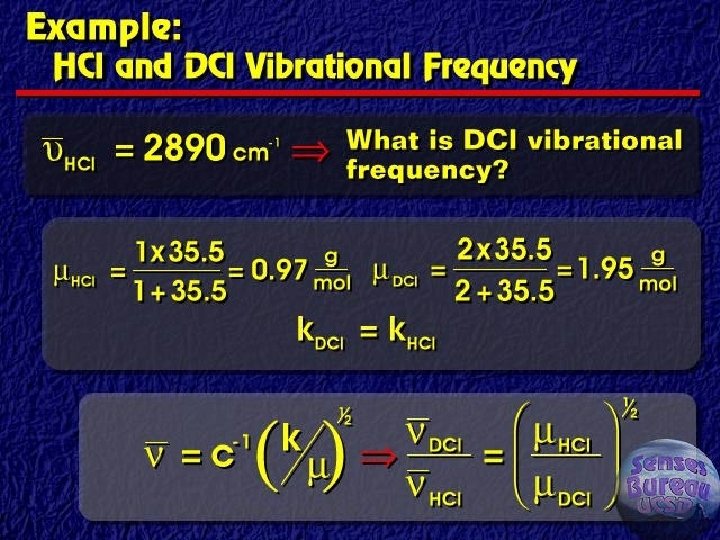
Classical vibrational frequency F = ma = m(d 2 y/dt 2) ► F=-ky ► Solution of differential equation ► § Y = A cos (2 pnt) § D 2 y/dt 2 = -4 p 2 n 2 A cos (2 pnt) § Reduced mass for two masses







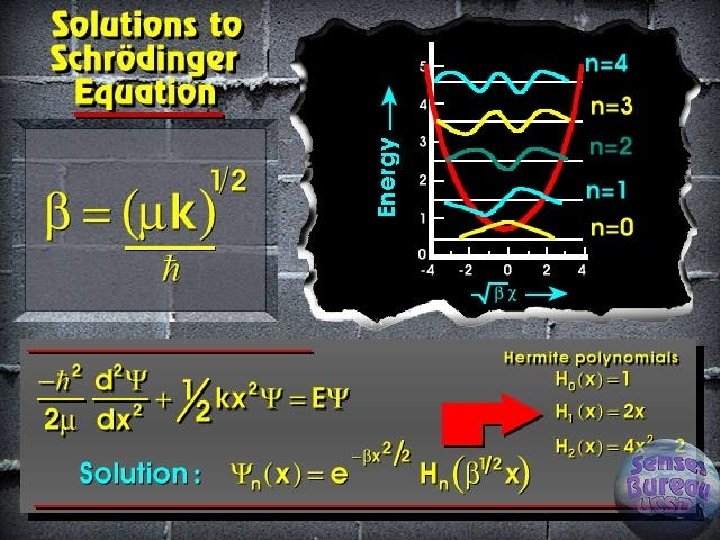
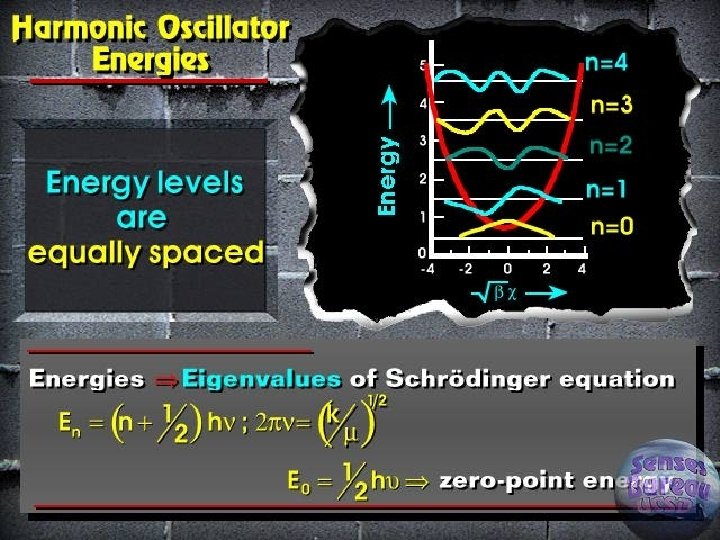
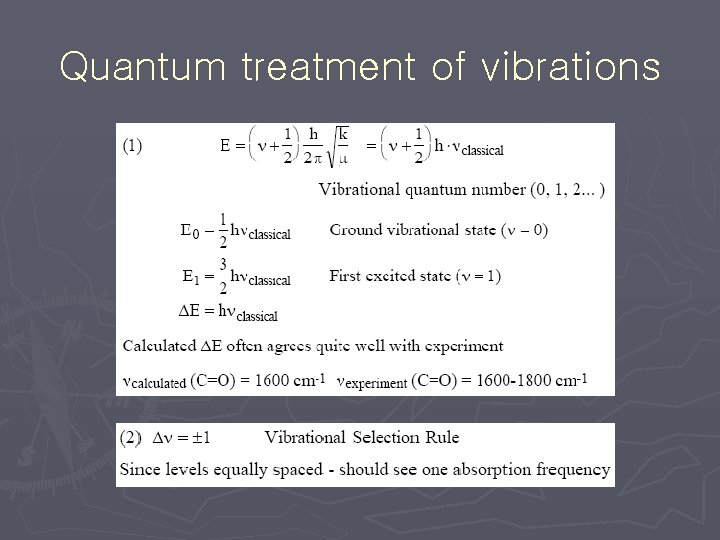
Quantum treatment of vibrations

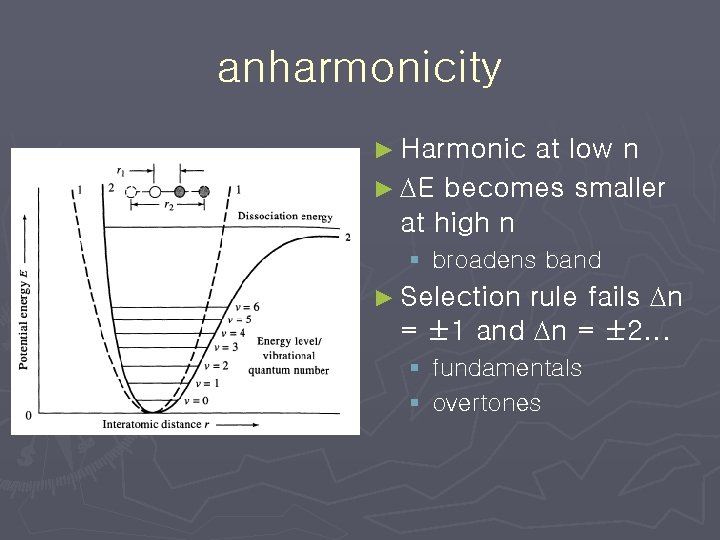
Anharmonic Oscillator ► Must modify harmonic oscillator potential for § electron repulsion ►steeper at small distances § dissociation ►bond breaks at large distances

anharmonicity ► Harmonic at low n ► DE becomes smaller at high n § broadens band rule fails Dn = ± 1 and Dn = ± 2. . . ► Selection § fundamentals § overtones

Vibrational Normal modes ► Number of possible vibrations in a polyatomic molecule § § § § ► 2 atoms (H 2) - 1 vibration (stretch n) 3 atoms (H 2 O) - 3 vibrations (n s, n as, s) 3 atoms (CO 2) - 4 vibrations (n s, n as, s, w) 4 atoms (H 2 CO) - 6 vibrations (n s, n as, s, w, r(CH 2) n(C=O)) 5 atoms. . . 3 N - 6 Non - linear molecule 3 N - 5 Linear molecule 3 N degrees of freedom for N atoms § 3 translation § 3(or 2) rotation – rotation about the bond axis is not possible § Orhters are "Normal modes"

Fewer experimental peaks ► Fewer peaks § Symmetry of the molecule § degenracy ► Energies of two or more vibrations are identical ► Or nearly identical § Undetectable low absorption intensity § Out of the instrumental detection range ► More peaks § Overtone § Combination bands

Applications of FT-IR ► Chemical Analysis: § Match spectra to known databases ► Identifying an unknown compound, Forensics, etc. § Monitor chemical reactions in-situ ► Structural ideas: § Can determine what chemical groups are in a specific compound ► Electronic Information: § Measure optical conductivity ► Determine if Metal, Insulator, Superconductor, Semiconductor § Band Gaps, Drude model

Vibrational coupling ► Coupling of different vibrations shifts frequencies ► Energy of a vibration is influenced by coupling ► Coupling likely when § § common atom in stretching modes common bond in bending+stretching modes similar vibrational frequencies ► Coupling not likely when § atoms separated by two or more bonds § symmetry inappropriate

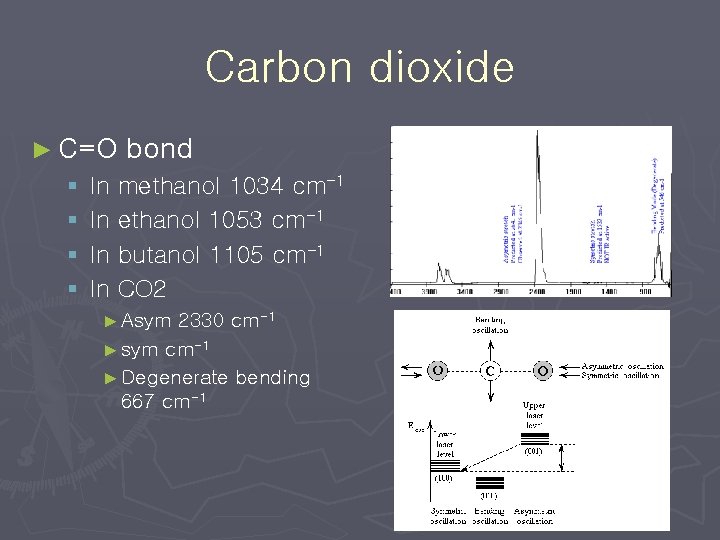
Carbon dioxide ► C=O bond § In methanol 1034 cm-1 § In ethanol 1053 cm-1 § In butanol 1105 cm-1 § In CO 2 2330 cm-1 ► sym cm-1 ► Degenerate bending 667 cm-1 ► Asym

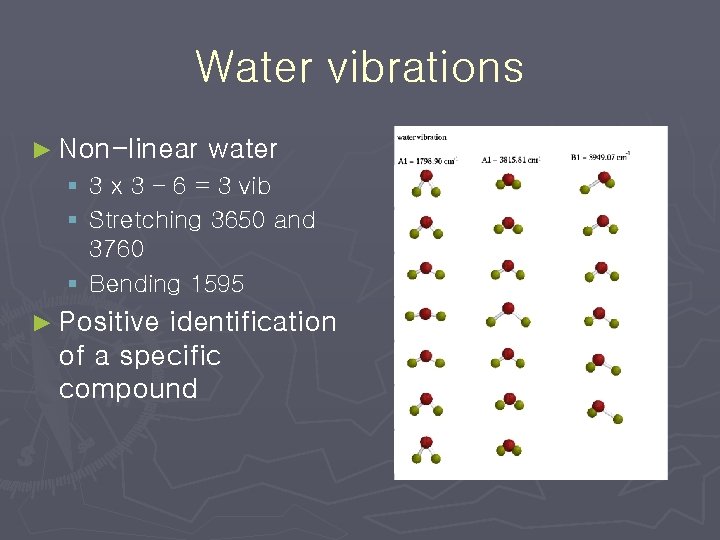
Water vibrations ► Non-linear water § 3 x 3 – 6 = 3 vib § Stretching 3650 and 3760 § Bending 1595 ► Positive identification of a specific compound

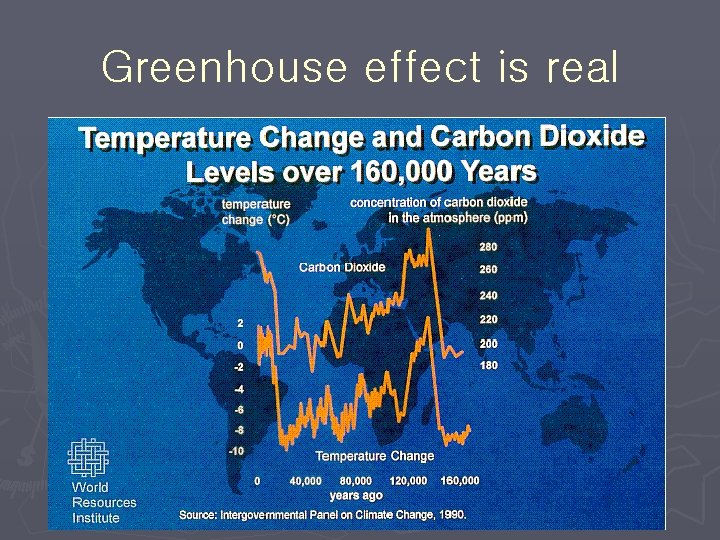
Global climate change Recent years have seen a huge rise in the number of abnormal weather events. ► Meteorologists agree that these exceptional conditions are signs that Global Climate Change is happening already. ► Scientists agree that the most likely cause of the changes are man-made emissions of the so-called "Greenhouse Gases" that can trap heat in the earth's atmosphere in the same way that glass traps heat in a greenhouse. ► Although there are six major groups of gases that contribute to Global Climate Change, the most common is Carbon Dioxide (CO 2). ►

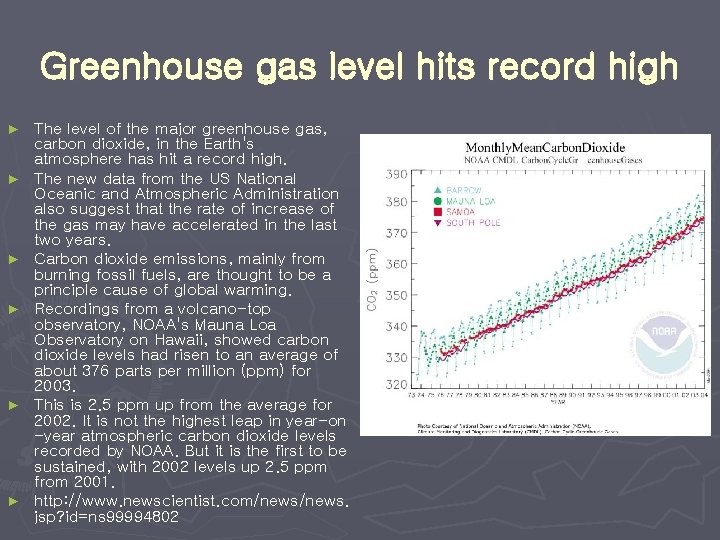
Greenhouse gas level hits record high ► ► ► The level of the major greenhouse gas, carbon dioxide, in the Earth's atmosphere has hit a record high. The new data from the US National Oceanic and Atmospheric Administration also suggest that the rate of increase of the gas may have accelerated in the last two years. Carbon dioxide emissions, mainly from burning fossil fuels, are thought to be a principle cause of global warming. Recordings from a volcano-top observatory, NOAA's Mauna Loa Observatory on Hawaii, showed carbon dioxide levels had risen to an average of about 376 parts per million (ppm) for 2003. This is 2. 5 ppm up from the average for 2002. It is not the highest leap in year-on -year atmospheric carbon dioxide levels recorded by NOAA. But it is the first to be sustained, with 2002 levels up 2. 5 ppm from 2001. http: //www. newscientist. com/news. jsp? id=ns 99994802

Greenhouse effect is real

The day after tomorrow

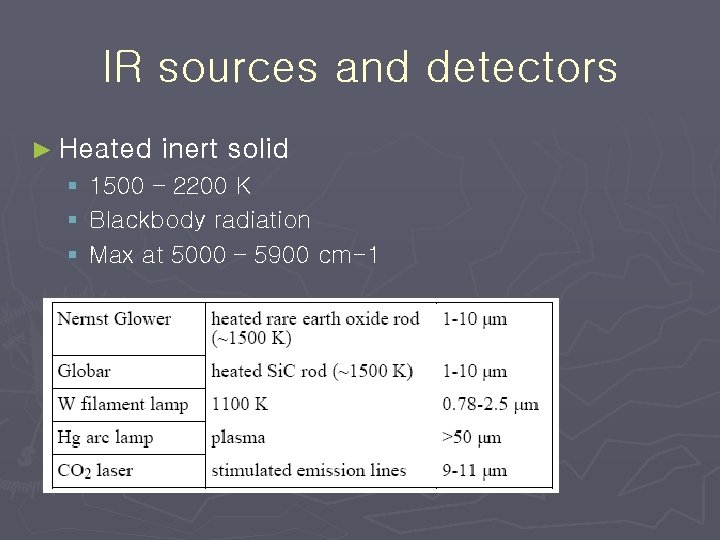
IR sources and detectors ► Heated inert solid § 1500 – 2200 K § Blackbody radiation § Max at 5000 – 5900 cm-1

Globar source ► Globar § § ► Silicon carbide rod Diameter 5 mm x length 50 mm Heated 1300 -1500 K Water cooling to prevent arcing Compare to Nernst glower § Spectral energies are comparable § At < 5 mm, Globar provides a greater output

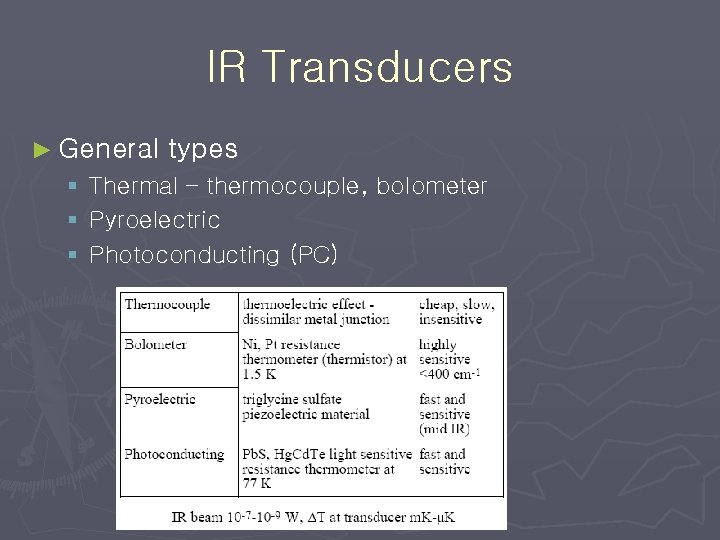
IR Transducers ► General types § Thermal – thermocouple, bolometer § Pyroelectric § Photoconducting (PC)

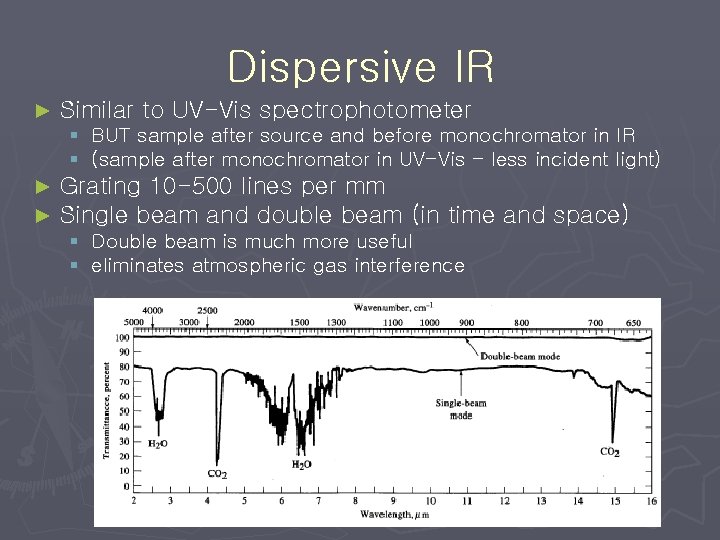
Dispersive IR ► Similar to UV-Vis spectrophotometer § BUT sample after source and before monochromator in IR § (sample after monochromator in UV-Vis - less incident light) ► ► Grating 10 -500 lines per mm Single beam and double beam (in time and space) § Double beam is much more useful § eliminates atmospheric gas interference

Bio-Rad FTS-40 FT-IR

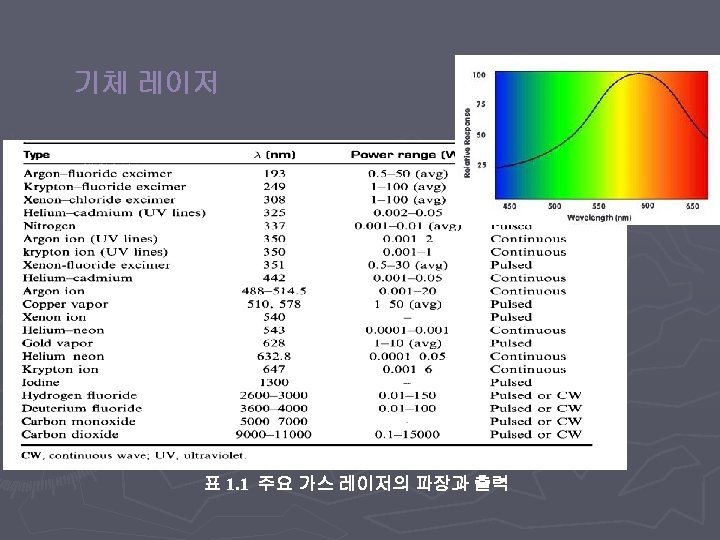
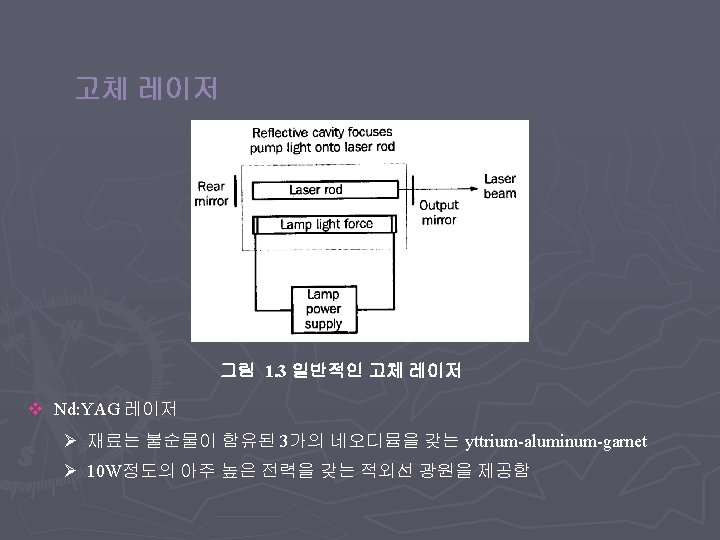
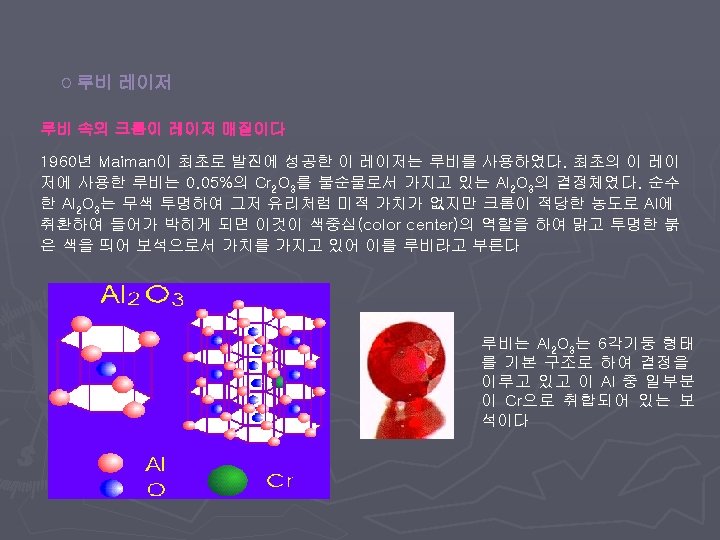
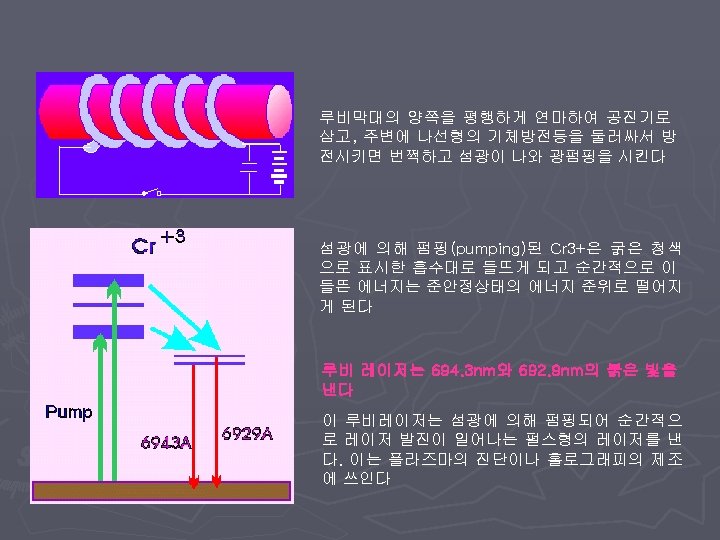
< Types of lasers > v Gas Laser v Solid State Laser v Semiconductor Laser

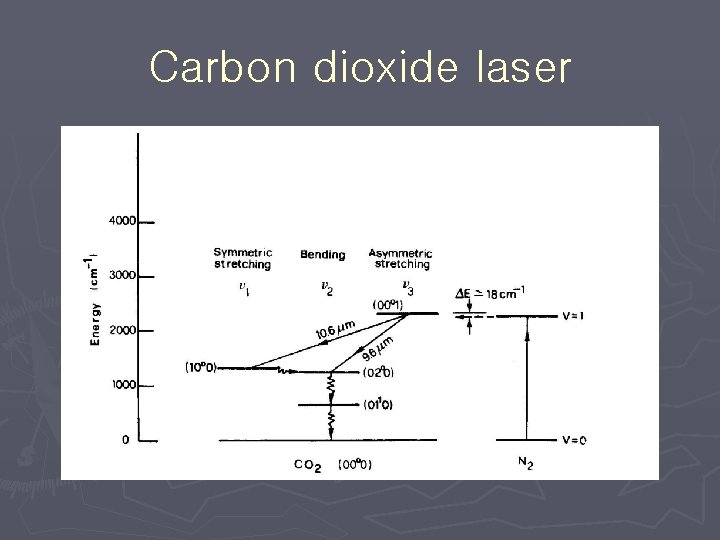
Carbon dioxide laser

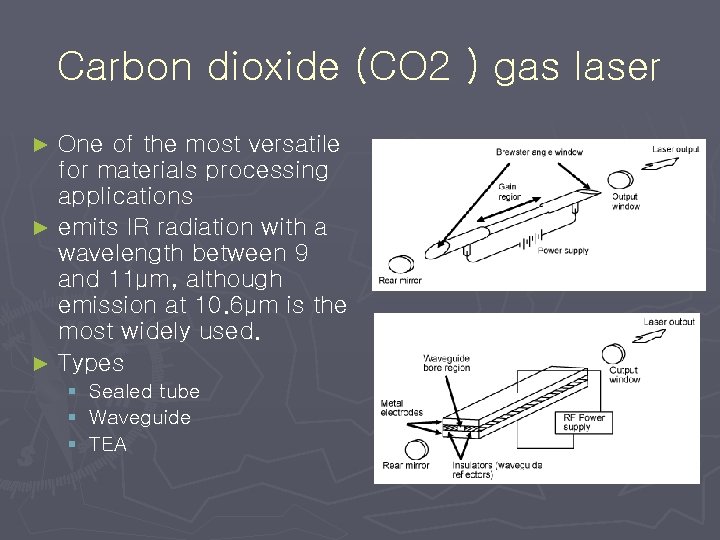
Carbon dioxide (CO 2 ) gas laser One of the most versatile for materials processing applications ► emits IR radiation with a wavelength between 9 and 11µm, although emission at 10. 6µm is the most widely used. ► Types ► § Sealed tube § Waveguide § TEA

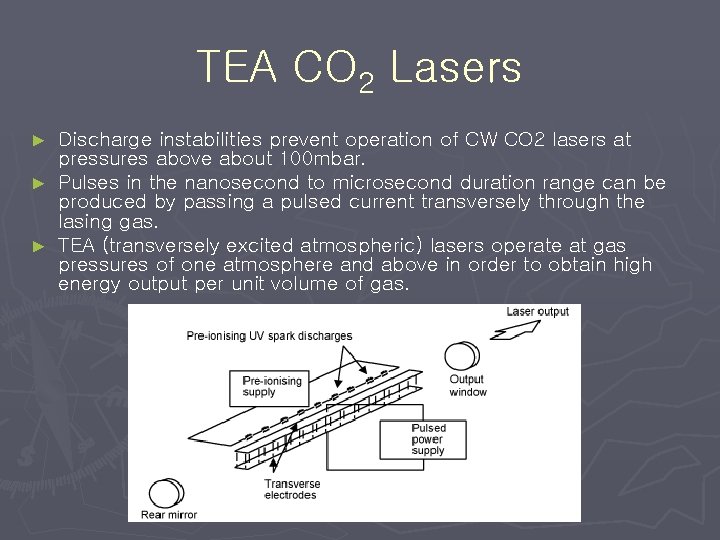
TEA CO 2 Lasers Discharge instabilities prevent operation of CW CO 2 lasers at pressures above about 100 mbar. ► Pulses in the nanosecond to microsecond duration range can be produced by passing a pulsed current transversely through the lasing gas. ► TEA (transversely excited atmospheric) lasers operate at gas pressures of one atmosphere and above in order to obtain high energy output per unit volume of gas. ►

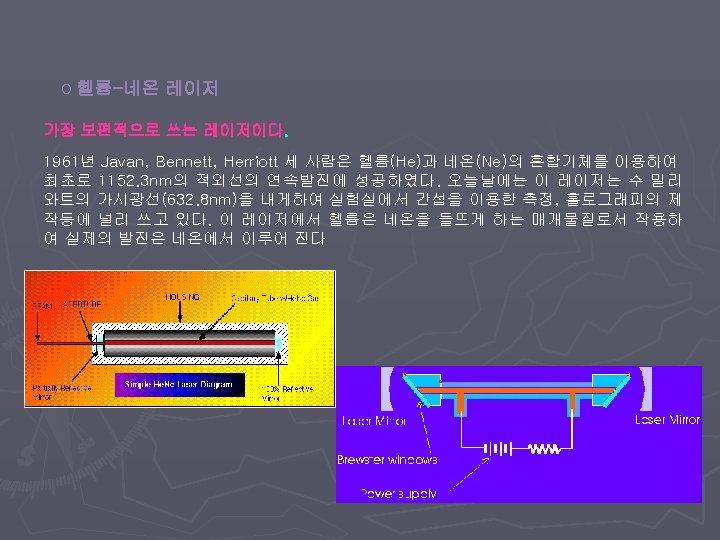
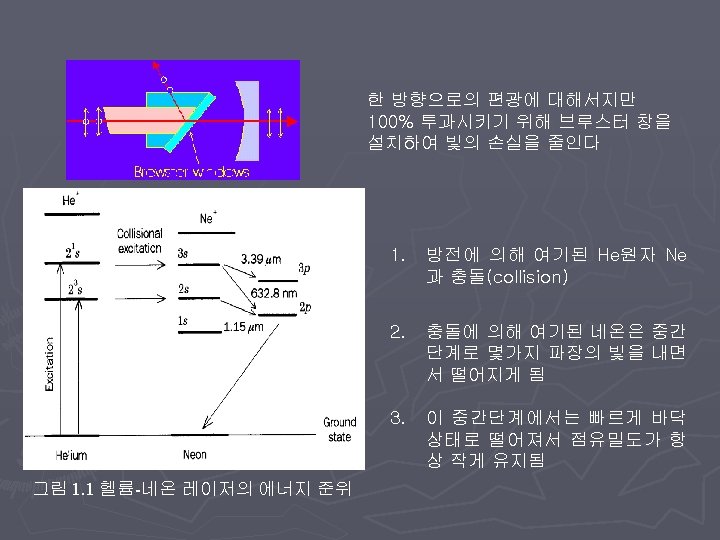
Home built CO 2 gas laser ► J&K Laser Productions












