The Ideal Gas Relationships The Combined Gas Law

The Ideal Gas Relationships & The Combined Gas Law Sections 11. 8 & 11. 9
Kinetic Molecular Theory (KMT) of Gases Set of statements (assumptions) that describes the interactions of gas molecules. 1. 2. 3. 4. 5. The molecules in a gas occupy no volume. There are no attractive or repulsive forces between the molecules. Gas molecules are constantly in motion, moving in straight lines until they collide. When two gas molecules collide, no energy is gained or lost during the collision. At any given temperature, the average kinetic energy of the particles in all gases is the same.
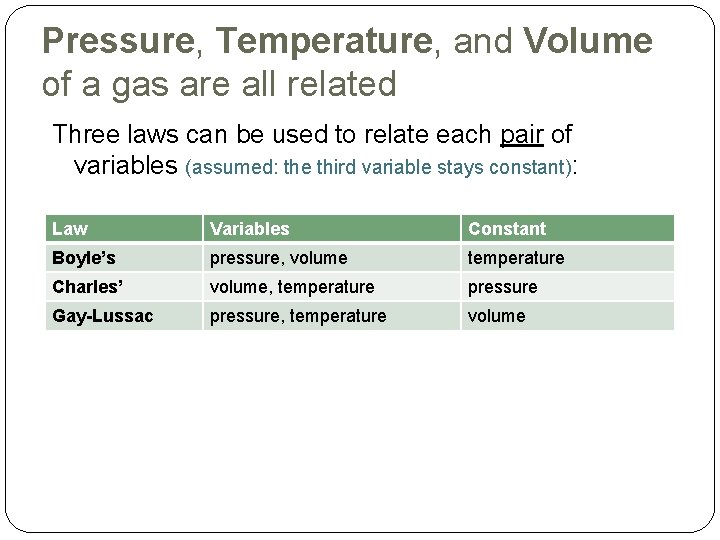
Pressure, Temperature, and Volume of a gas are all related Three laws can be used to relate each pair of variables (assumed: the third variable stays constant): Law Variables Constant Boyle’s pressure, volume temperature Charles’ volume, temperature pressure Gay-Lussac pressure, temperature volume
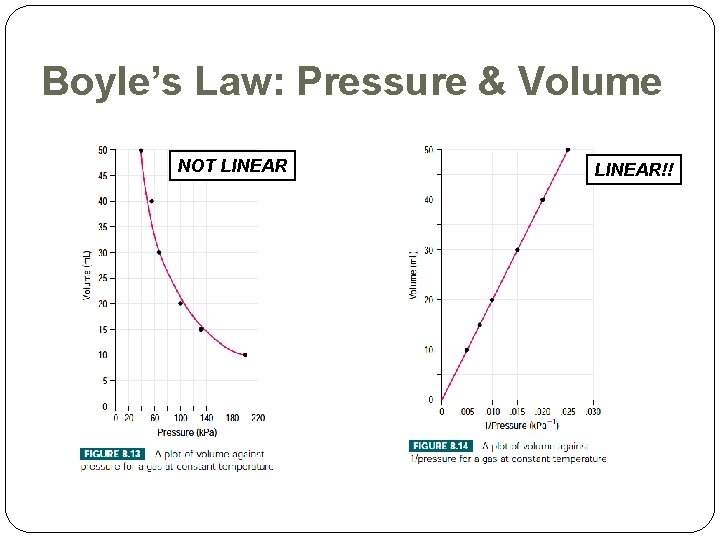
Boyle’s Law: Pressure & Volume NOT LINEAR!!
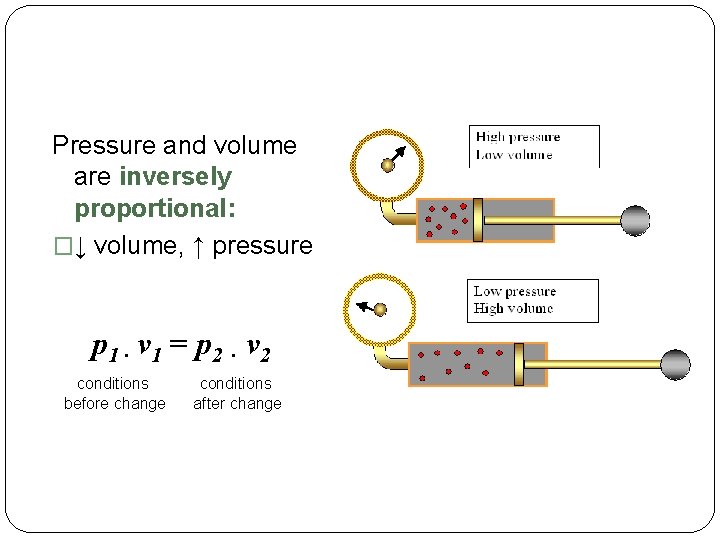
Pressure and volume are inversely proportional: �↓ volume, ↑ pressure p 1 · v 1 = p 2 · v 2 conditions before change conditions after change
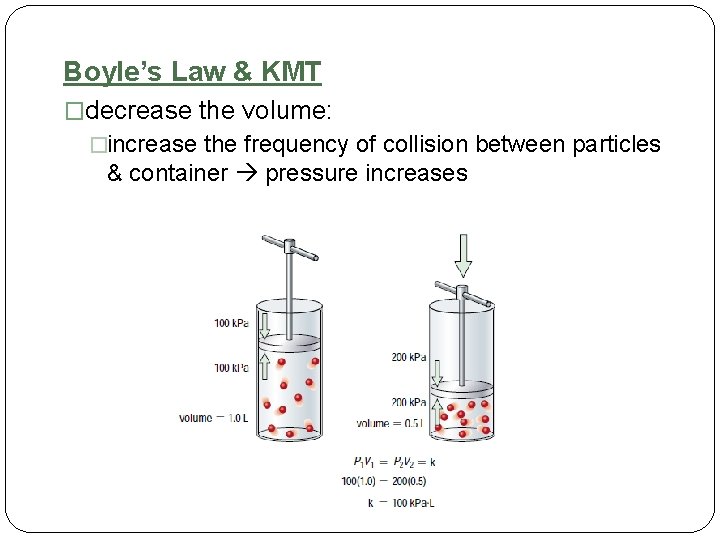
Boyle’s Law & KMT �decrease the volume: �increase the frequency of collision between particles & container pressure increases


Example 1 A balloon is filled to a volume of 5. 0 L in Vancouver at a pressure is 101 k. Pa. It is taken to Banff, where patm = 91 k. Pa. a. Will the balloon’s volume be larger or smaller in Banff? b. Suppose the temperature is the same in both places. What will be the new volume? V = 5. 5 L
Example 2 A certain mass of gas in a 2. 00 -L container has a pressure of 164 k. Pa. Calculate the new pressure of the gas if the volume of the container is reduced to 1. 00 L. P= 328 k. Pa
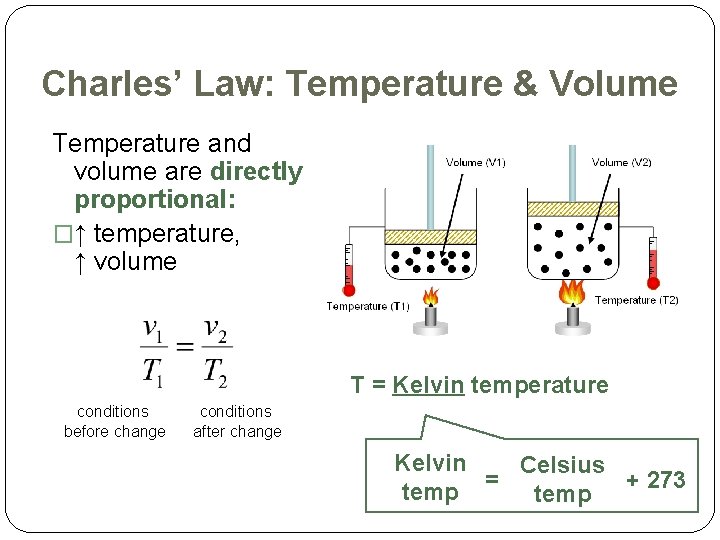
Charles’ Law: Temperature & Volume Temperature and volume are directly proportional: �↑ temperature, ↑ volume T = Kelvin temperature conditions before change conditions after change Kelvin Celsius = + 273 temp
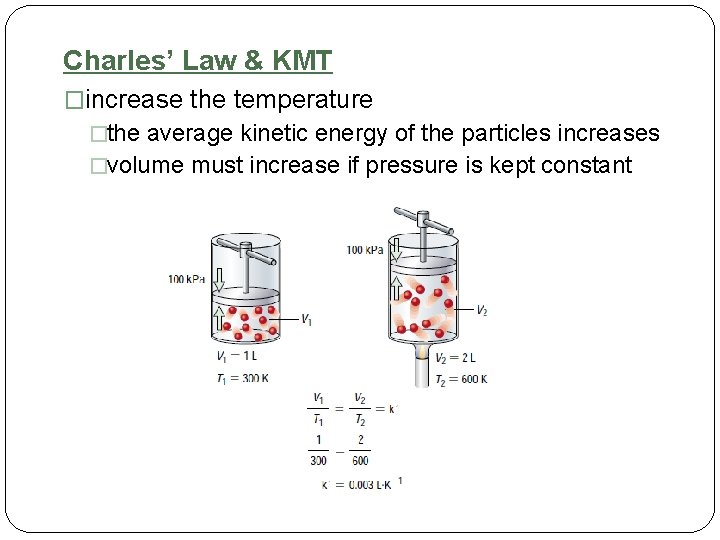
Charles’ Law & KMT �increase the temperature �the average kinetic energy of the particles increases �volume must increase if pressure is kept constant
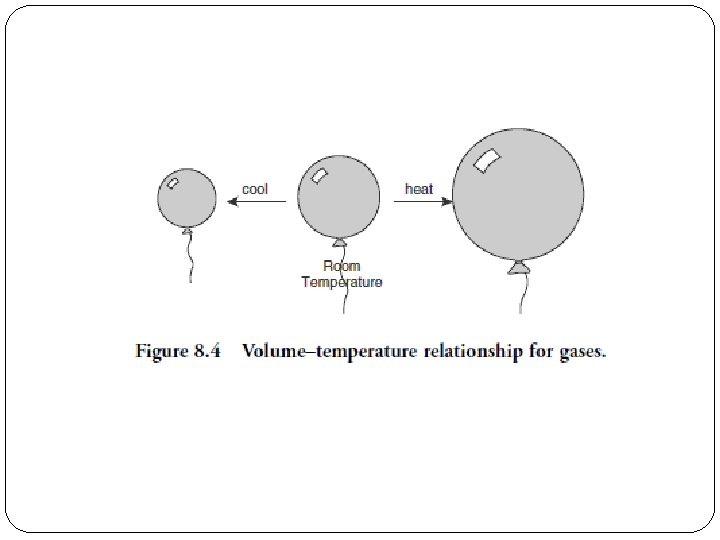

Example 3 A balloon is filled with helium gas to a volume of 1. 20 L at a temperature of 15°C. If pressure remains constant, but the temperature rises to 30°C, what will be the new volume? V = 1. 26 L

Example 4 A balloon contains 5. 00 L of air at 25°C. Deduce at what temperature the balloon will shrink to a volume of 4. 75 L. Assume constant pressure. T = 274 K = 1 °C
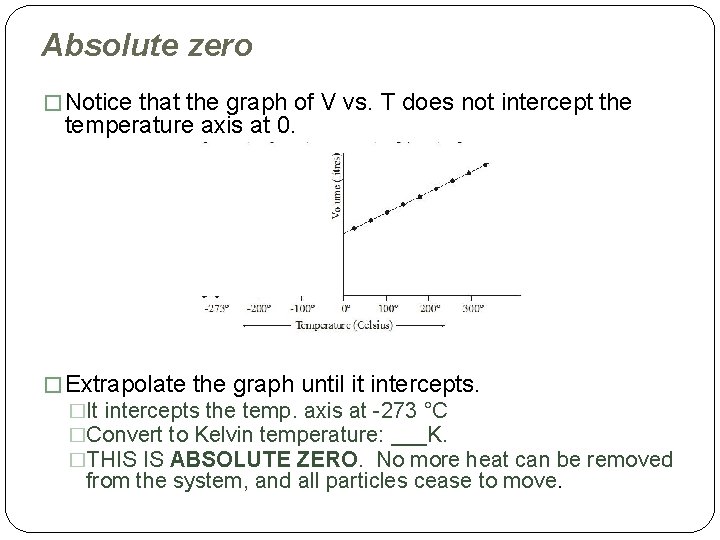
Absolute zero � Notice that the graph of V vs. T does not intercept the temperature axis at 0. � Extrapolate the graph until it intercepts. �It intercepts the temp. axis at -273 °C �Convert to Kelvin temperature: ___K. �THIS IS ABSOLUTE ZERO. No more heat can be removed from the system, and all particles cease to move.
Homework �Boyle’s Law and Charles’ Law �Pg. 549 #1, 2 �Pg. 553 #1 -6 �Worksheet
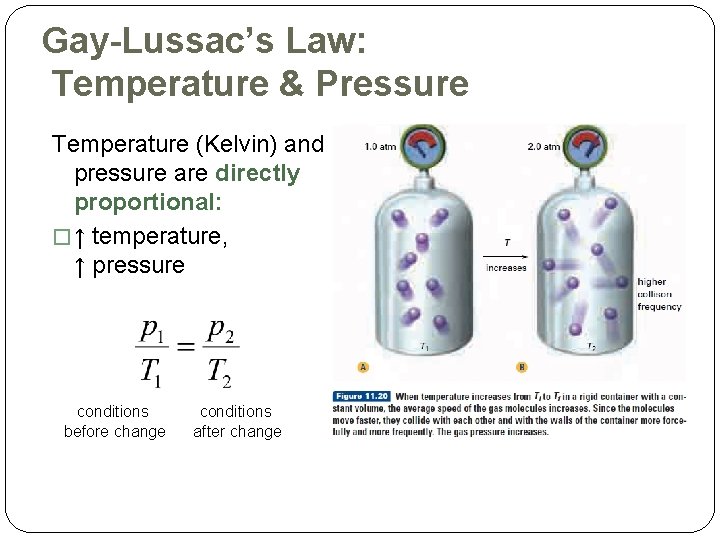
Gay-Lussac’s Law: Temperature & Pressure Temperature (Kelvin) and pressure are directly proportional: � ↑ temperature, ↑ pressure conditions before change conditions after change
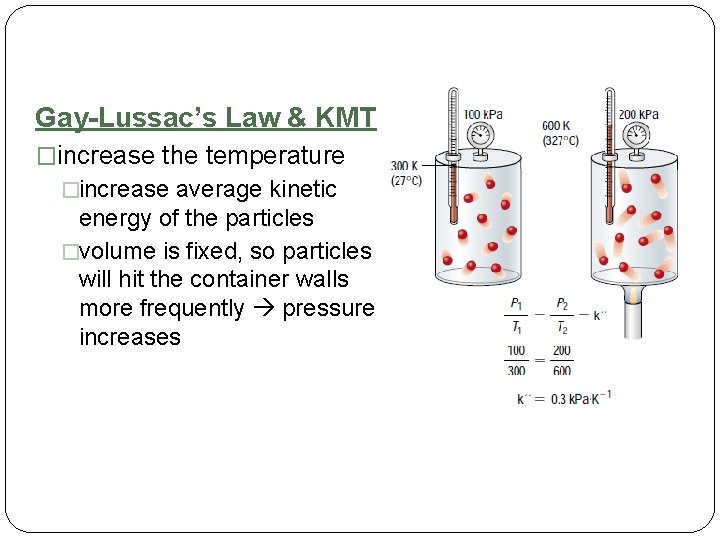
Gay-Lussac’s Law & KMT �increase the temperature �increase average kinetic energy of the particles �volume is fixed, so particles will hit the container walls more frequently pressure increases
Example 5 At a temperature of 10°C, a container is filled with gas at a pressure of 225 k. Pa. What will the pressure be if the container is placed in the hot sun to reach a temperature of 42°C? P = 250 k. Pa
Example 6 A glass vessel that can only withstand a maximum internal pressure of 225 k. Pa is filled with gas at 21°C and 100. 0 k. Pa, and then heated. At what temperature will the vessel burst? T = 662 K = 389 °C
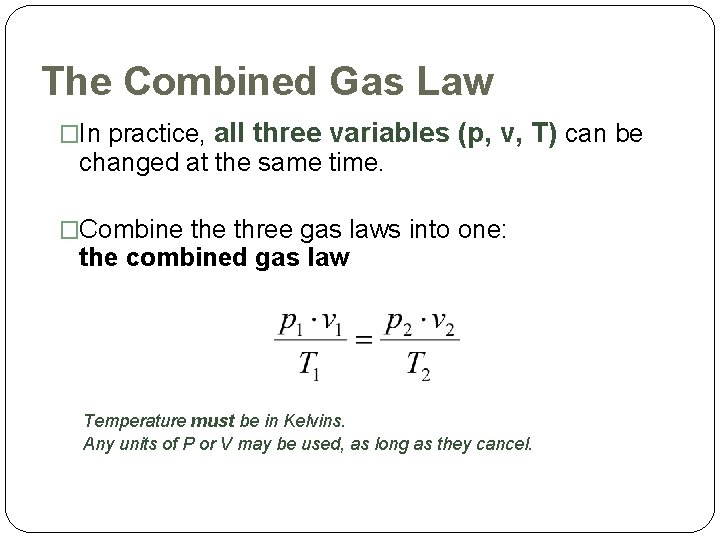
The Combined Gas Law �In practice, all three variables (p, v, T) can be changed at the same time. �Combine three gas laws into one: the combined gas law Temperature must be in Kelvins. Any units of P or V may be used, as long as they cancel.
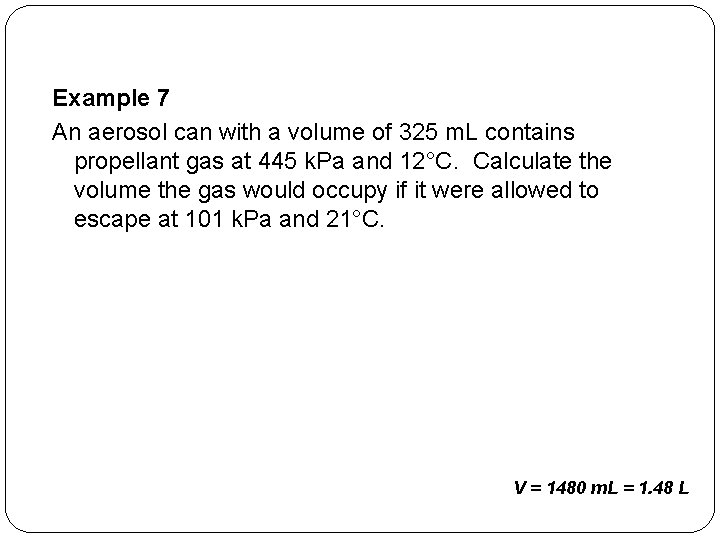
Example 7 An aerosol can with a volume of 325 m. L contains propellant gas at 445 k. Pa and 12°C. Calculate the volume the gas would occupy if it were allowed to escape at 101 k. Pa and 21°C. V = 1480 m. L = 1. 48 L
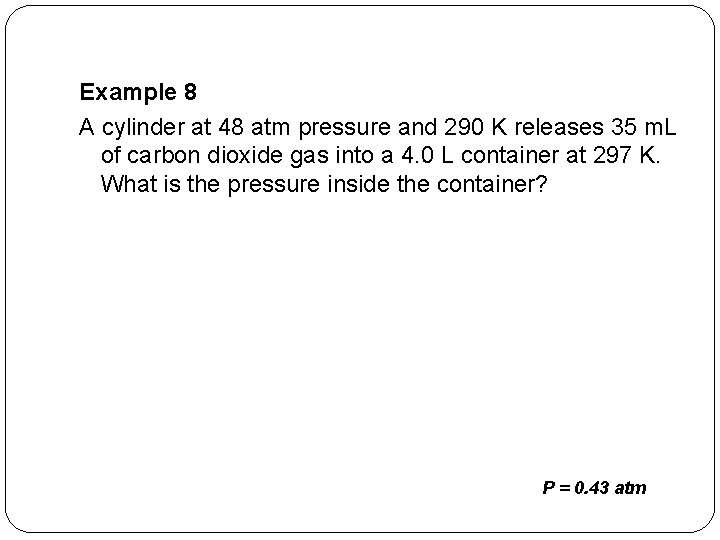
Example 8 A cylinder at 48 atm pressure and 290 K releases 35 m. L of carbon dioxide gas into a 4. 0 L container at 297 K. What is the pressure inside the container? P = 0. 43 atm

Homework �Worksheets: �Gay-Lussac’s law �Combined gas law
- Slides: 24