The ICU as a Learning Healthcare Environment Bumps

The ICU as a Learning Healthcare Environment: Bumps in the Road from Quality Improvement to Comparative Effectiveness Research Todd W. Rice, MD, MSc Associate Professor of Medicine Division of Allergy, Pulmonary, and Critical Care Medicine Vanderbilt University School of Medicine Ethical, Legal and Social Implications of Learning Health Systems Symposium University of Michigan Learning Health Sciences November 18, 2016

Disclosures • I have no financial conflicts of interest relevant to the material in this presentation

Objectives • Describe how Vanderbilt transitioned from QI projects in the ICU to using the ICU as our learning healthcare environment • Describe three different comparative effectiveness studies conducted in the Vanderbilt ICU learning healthcare environment • Briefly describe some of the obstacles encountered along the way

Chlorhexidine Bathing in the ICU The QI to LHS Transition Study
Background • Healthcare-associated infections – Most frequent complication of hospitalized patients – Associated with increased morbidity, mortality, healthcare costs • Chlorhexidine gluconate – Broad spectrum, topical antimicrobial – Used to prevent infections • Catheter insertion bundles, preoperative skin preparation • Preoperative bathing, showers, hand hygiene

Evidence supporting chlorhexidine bathing • Multiple quasi-experimental studies – Inconsistent reductions in MDRO colonization, CLABSI, VAP, or C. difficile • One small cluster-randomized trial – Reduced BSI • Bleasdale, et al. Arch Intern Med. 2007 • One large, cluster-randomized trial – Reduced MDRO acquisition, HA-BSI, and CLABSI • Climo, et al. NEJM. 2013

Hypothesis • Daily bathing of the skin with chlorhexidine will reduce the incidence of healthcareassociated infections in critically ill patients

Design • Design: cluster randomized, multiple crossover trial • Setting: five ICUs at a 1, 025 bed tertiary medical center in Nashville, Tennessee from July 2012 through July 2013 • Participants: Inclusion: all patients admitted to participating ICUs Exclusion: burns, TEN/SJS, known chlorhexidine allergy • IRB approval with waiver of consent

Intervention • All patients bathed daily with no-rinse cloths: – impregnated with chlorhexidine – without chlorhexidine • All other infection control procedures were performed according to usual practice of each ICU for the duration of the study
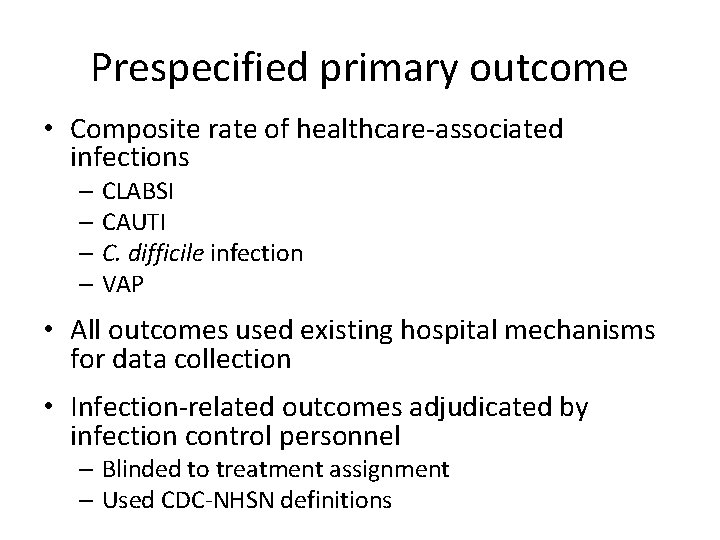
Prespecified primary outcome • Composite rate of healthcare-associated infections – CLABSI – CAUTI – C. difficile infection – VAP • All outcomes used existing hospital mechanisms for data collection • Infection-related outcomes adjudicated by infection control personnel – Blinded to treatment assignment – Used CDC-NHSN definitions
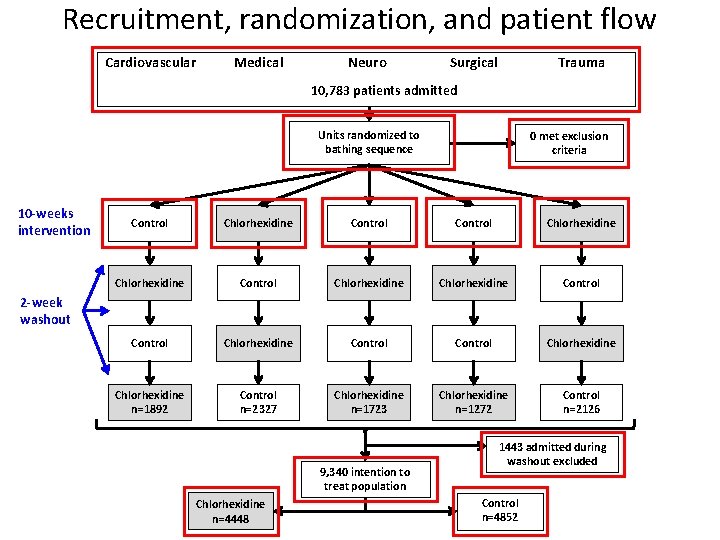
Recruitment, randomization, and patient flow Cardiovascular Medical Neuro Trauma Surgical 10, 783 patients admitted Units randomized to bathing sequence 10 -weeks intervention 0 met exclusion criteria Control Chlorhexidine Chlorhexidine Control Chlorhexidine Control Chlorhexidine n=1892 Control n=2327 Chlorhexidine n=1723 Chlorhexidine n=1272 Control n=2126 2 -week washout 9, 340 intention to treat population Chlorhexidine n=4448 1443 admitted during washout excluded Control n=4852

MJ Noto and coauthors Chlorhexidine Bathing and Health Care–Associated Infections: A Randomized Clinical Trial Published online January 20, 2015 Available at jama. com and on The JAMA Network Reader at mobile. jamanetwork. com

Conclusion • These findings do not support daily bathing of critically ill patients with chlorhexidine.

Isotonic Solutions and Major Adverse Renal Events Trial (SMART) Hypothesis: Use of “physiologicallybalanced” isotonic crystalloids compared to 0. 9% saline in ICU patients will decrease the incidence of death, dialysis, and persistent renal dysfunction.
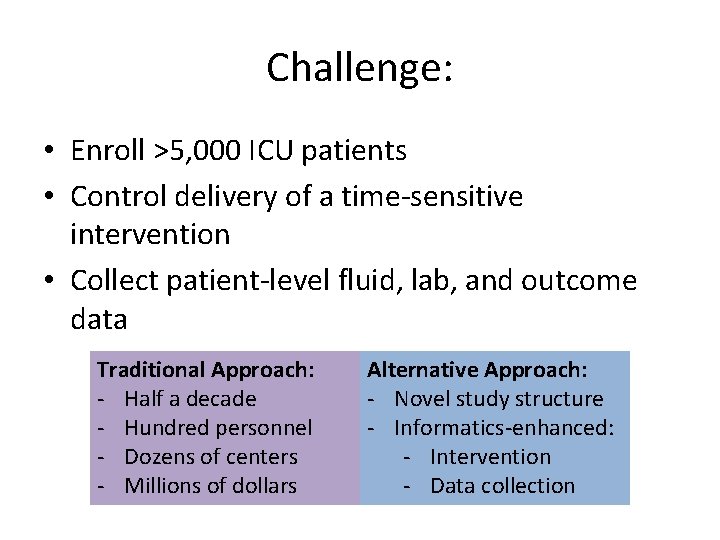
Challenge: • Enroll >5, 000 ICU patients • Control delivery of a time-sensitive intervention • Collect patient-level fluid, lab, and outcome data Traditional Approach: - Half a decade - Hundred personnel - Dozens of centers - Millions of dollars Alternative Approach: - Novel study structure - Informatics-enhanced: - Intervention - Data collection
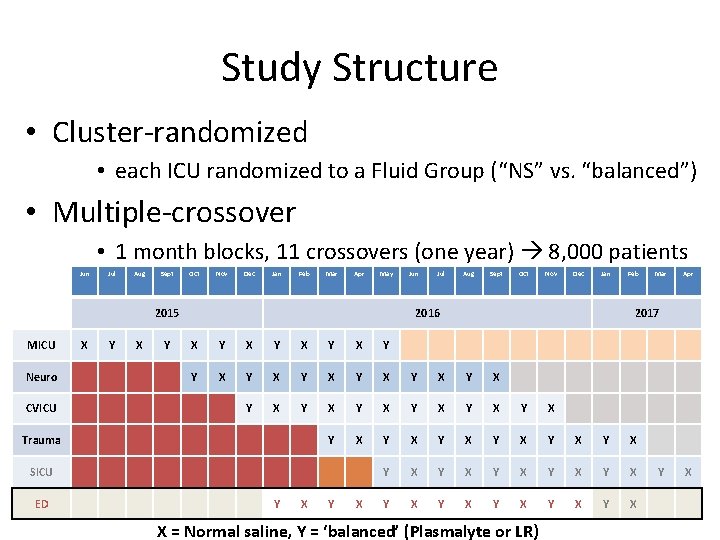
Study Structure • Cluster-randomized • each ICU randomized to a Fluid Group (“NS” vs. “balanced”) • Multiple-crossover • 1 month blocks, 11 crossovers (one year) 8, 000 patients Jun Jul Aug Sept Oct Nov Dec Jan Feb Mar Apr May 2015 MICU Neuro CVICU X Y Jul Aug Sept Oct Nov Dec Jan 2016 Feb Y X Y X Y X Y X Y X Y X Y X Y X SICU Y X Mar Apr 2017 X Trauma ED Jun X = Normal saline, Y = ‘balanced’ (Plasmalyte or LR) Y X
Step 1 Delivery of the Intervention

Delivery of the Intervention If assigned to Normal Saline, can bypass for: - specific attending request Step 3 If assigned to Balanced Fluid can bypass for: - hyperkalemia - brain injury - specific attending request Pharmacy stocks unit preferentially with assigned fluid

Data Collection • Electronic Extraction (Bioinformatics) – Baseline – On-study – Outcomes • In-hospital death • New Renal Replacement Therapy • Persistent Renal Dysfunction (discharge Cr ≥ 200% baseline)


Video vs. Direct Laryngoscopy in Critically Ill Patients Patient Level Randomization LHS

FELLOW Study Design • Open-label, parallel-group, randomized trial • Video Laryngoscopy vs. Direct Laryngoscopy • Inclusion: – 18 or older – being intubated by PCCM fellow • Exclusion: – awake intubation – too emergent to open study envelope – specific laryngoscope required
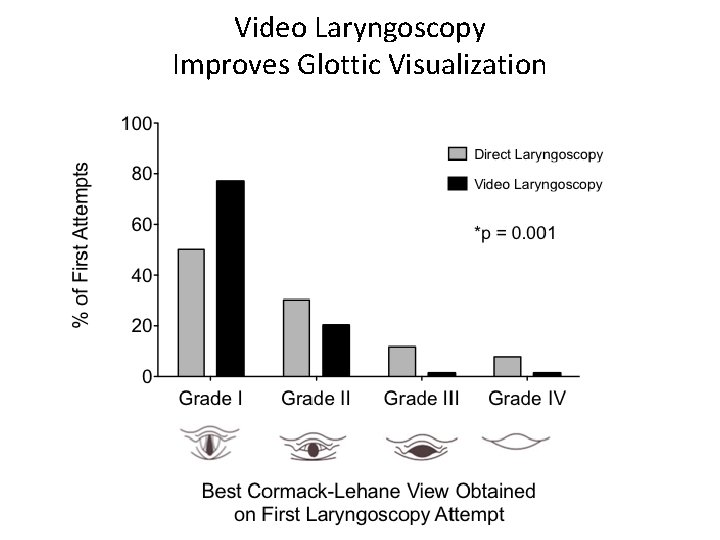
Video Laryngoscopy Improves Glottic Visualization
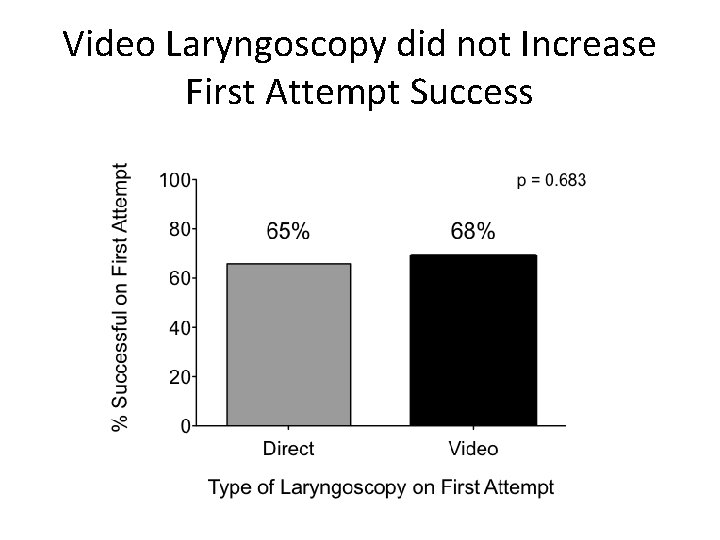
Video Laryngoscopy did not Increase First Attempt Success
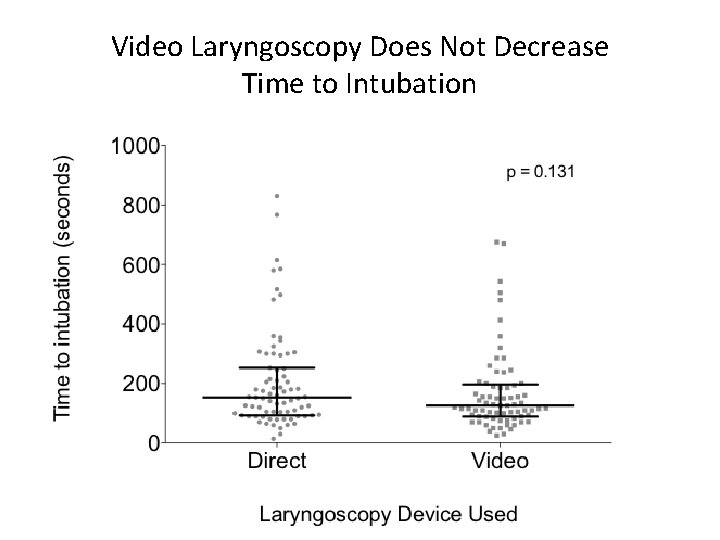
Video Laryngoscopy Does Not Decrease Time to Intubation


Challenges Encountered / Overcome 1. Informed Consent / Waiver of Informed Consent 2. Study Design: Cluster, Cluster crossover, Individual Randomization 3. Delivery of the Intervention 4. Collecting outcomes w/in the LHS

Informed Consent • Waiver of Informed Consent – Minimal Risk – Waiver does not adversely affect the rights or welfare of the participants – Consent Impracticable – When appropriate, participants will be provided with additional pertinent information after participation – Minimal risk: - demonstrated that the research arms occur in practice, and appear to be arbitrary in their delivery; option for primary team opt out in IV fluids and intubation – Consent impracticable – bathing of others may influence neighbors’ infections; delivery of IV fluids prior to being able to obtain consent; 90+% of time, no consent for intubation

Study Design • CHX bathing – Risk for crossover if both cloth types available in single ICU – Bathing of others may affect neighbor’s infections – 2: 1 nursing assignments may result in nurse with patient in each arm • IV Fluids – Cluster due to need for ready access in Pyxis, pharmacy control – Research question: Does changing the type of IV fluid available in the ICU improve patient outcomes? • Intubation– – Individualized randomization with envelope in workroom
Delivery of the Intervention • CHX bathing – Controlled supply of cloths to the ICUs • IV Fluids – Via Pharmacy, controlled IV fluid type delivered to the ICU – Through electronic ordering, set up prompts to order fluid which is the assigned for that month • Intubation– – Both devices readily available

Collecting Outcomes w/in LHS • CHX bathing – Outcomes already collected by hospital (HAI, mortality, LOS) • IV Fluids – Automated extraction from the EMR – Some outcomes already collected by hospital • Intubation– – Data collected on randomization sheet in the envelopes – Longer term outcomes collected from EMR


Summary • The ICU is an environment rich for LHS • Waiver of consent, study design, collection of data, and outcomes are study (i. e. intervention) specific • Working closely with all aspects of the LHS can facilitate pragmatic studies (IRB, nursing, pharmacy, bioinformatics, etc)
- Slides: 33