The HPV Vaccine A Clinical Update Karen Soren























- Slides: 41

The HPV Vaccine: A Clinical Update Karen Soren, MD Director, Adolescent Medicine Associate Clinical Professor, Pediatrics & Public Health College of Physicians and Surgeons Columbia University Medical Center

Learning Objectives • To review the epidemiology and clinical significance of infection with human papillomavirus (HPV) • To discuss recommendations for vaccination with HPV vaccine in girls, young adult women and males • To discuss controversies involving the HPV vaccine with respect to parental acceptance, state mandates, side effects and use in males

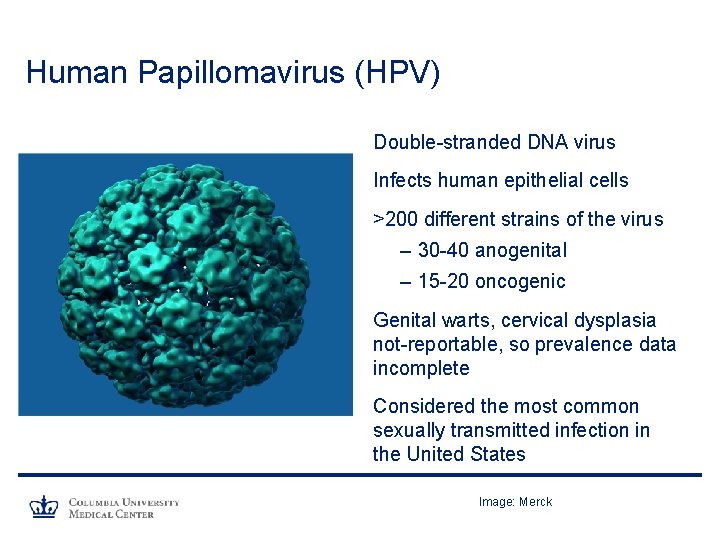
Human Papillomavirus (HPV) Double-stranded DNA virus Infects human epithelial cells >200 different strains of the virus – 30 -40 anogenital – 15 -20 oncogenic Genital warts, cervical dysplasia not-reportable, so prevalence data incomplete Considered the most common sexually transmitted infection in the United States Image: Merck

Epidemiology of HPV Infection • 10% worldwide prevalence (highest in Africa) • 20 million in US currently infected with anogenital strain • 5. 5 million/yr in US acquire new genital HPV infection • 3/4 of infections occur in 15 - 24 year olds • Among women 14 -59, overall HPV prevalence – 27% • Almost 40% of sexually active 14 -19 year old girls and 50% of sexually active 20 -24 year olds infected • Prospective study of female college students: 26% infected at baseline; of those who were negative, 43% acquired HPV infection over 3 years NHANES data 2003 -2004 Dunne et al, JAMA 2007 Bierman et al, NEJM 1998 CDC (MMWR) 2007

Human Papilloma Virus- Natural History • Over half of sexually active women & men infected with HPV at some point in their lives • Most HPV infections are asymptomatic and transient (~91% resolve without treatment in 2 years) • Reactivation or re-infection possible • In some individuals, HPV infections result in genital warts or Pap test abnormalities • Persistence of HPV infection (with high-risk subtypes) associated with a variety of anogenital cancers

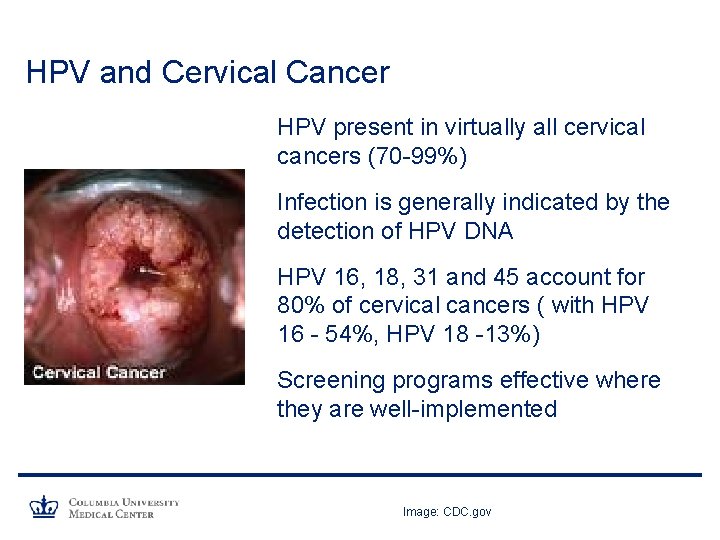
HPV and Cervical Cancer HPV present in virtually all cervical cancers (70 -99%) Infection is generally indicated by the detection of HPV DNA HPV 16, 18, 31 and 45 account for 80% of cervical cancers ( with HPV 16 - 54%, HPV 18 -13%) Screening programs effective where they are well-implemented Image: CDC. gov

HPV and Cervical Cancer US Statistics: – In 2009, estimated 11, 270 new cases of invasive cervical cancer with 4, 070 deaths – Median age of diagnosis – 48 years – Prevalence greatest in minority women (Hispanics> African Americans> Caucasians) National Cancer Institute, 2010 update SEER (Surveillance, Epidemiology, and End Results) data, NCI, 2007

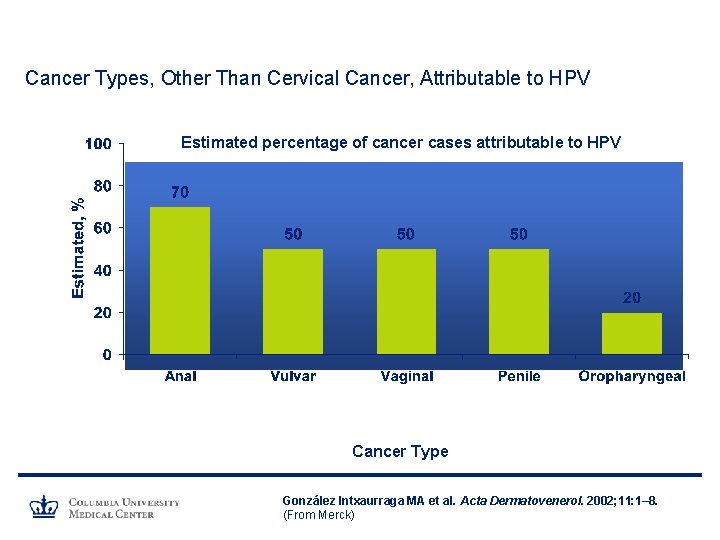
Cancer Types, Other Than Cervical Cancer, Attributable to HPV Estimated percentage of cancer cases attributable to HPV Cancer Type González Intxaurraga MA et al. Acta Dermatovenerol. 2002; 11: 1– 8. (From Merck)

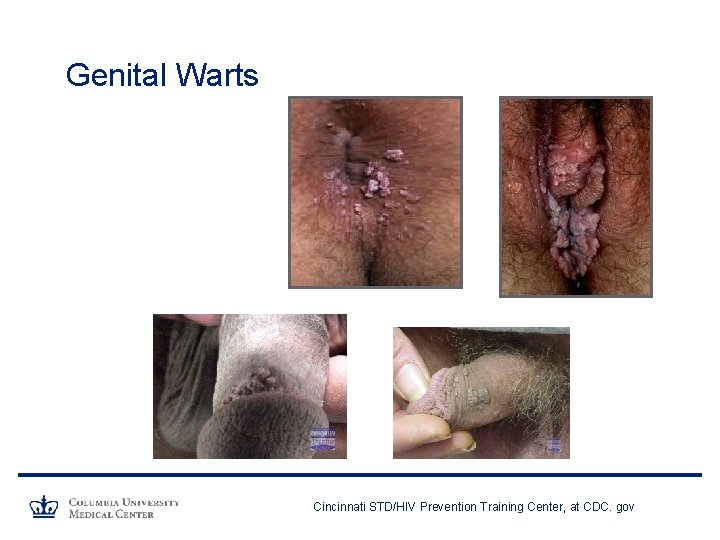
HPV and Anogenital Warts • HPV 6 and 11 responsible for >90% of anogenital warts • In 2006, ~ 420, 000 reported cases of genital warts in US • Prevalence: 1. 5% - 13% • Topical /surgical therapies available • Treatment can be painful and embarrassing • Up to 1/3 of genital warts may regress spontaneously within 3 months • Recurrence rates vary greatly • Significant psychological burden

Genital Warts Cincinnati STD/HIV Prevention Training Center, at CDC. gov

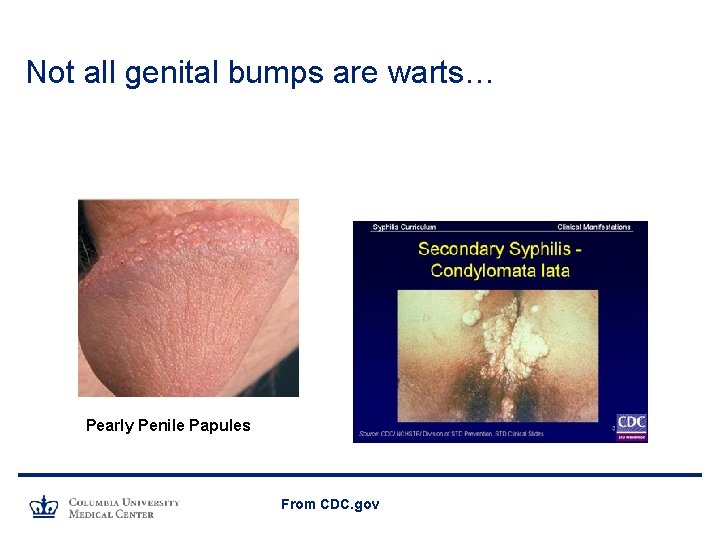
Not all genital bumps are warts… Pearly Penile Papules From CDC. gov

Scenario 1 • You offer the HPV vaccine to a 14 year old patient who you are seeing for a regular check-up. Her mother tells you that she is concerned that if you give the vaccine, her daughter will interpret that as permission to become sexually active. She also feels that the vaccine is still too new and may have serious side effects.

Scenario 2 • A sexually active 16 year old girl requests the HPV vaccine while seeing you in order to get birth control. Her mother does not know she is here today and is unaware of her daughter's sexual activity or use of contraception. Can you give her the vaccine without informing the parent?

Scenario 3 • A 17 year old boy asks about the vaccine against genital warts and wants to know if you recommend it. His mother looks horrified – she says she thought that the vaccine was for girls only and was primarily a vaccine that protected against cervical cancer.

Common Parental Questions and Concerns • How safe is the vaccine? What are the side effects? • If I vaccinate my child, is she more likely to become sexually active? • When should she get this vaccine – isn’t it better to wait until she is older? • Is my child allowed to get the HPV vaccine without my permission?

More Questions… • If someone is currently infected with HPV, will the vaccine treat it? • What happens if you cannot come back on time for the second and third injections? • Will a woman still need Pap screening if she is vaccinated against the HPV virus? • Should boys be vaccinated against HPV? What is a permissive recommendation?

HPV Vaccine Gardasil® (Merck) – FDA approved 6/06 – Quadrivalent vaccine (HPV 4) – Uses virus-like particles, recombinant L 1 capsid proteins of individual HPV types – Adjuvant – aluminum hydrophosphate sulfate – Protects against HPV 6, 11 (75 -90% genital warts) and 16, 18 (70% cervical cancer) – Indicated for girls and women 9 - 26 years of age – Schedule: 0, 2 and 6 months – Protection demonstrated for at least 5 years

HPV Vaccine Cervarix ® (Glaxo. Smith. Kline) – FDA approved in 10/09 – Bivalent vaccine (HPV 2) – Uses virus-like particles, recombinant L 1 capsid proteins of individual HPV types – Uses novel proprietary aluminum- based adjuvant – Protects against HPV 16 and 18 – Indicated for women 10 -25 (26 by ACIP) – Schedule: 0, 1 and 6 months – Protection demonstrated for at least 6. 4 years

HPV Vaccine Efficacy- HPV 4 (Gardasil) Clinical trials demonstrated: – 98% efficacy in preventing cervical pre-cancers caused by targeted HPV types in women uninfected at baseline – Girls who have not already been infected with any of the 4 sub-types of HPV get the most benefit from vaccine (44% efficacy in all women irrespective of baseline HPV status) – Vaccine nearly 100% efficacious in preventing vulvar/ vaginal pre-cancers and genital warts caused by targeted HPV types – May offer cross-protection against HPV type 31 Future II Study Group, NEJM, 2007

HPV Vaccine Efficacy- HPV 2 (Cervarix) Clinical trials demonstrated: – Vaccine may be more immunogenic than HPV 4 with higher post-vaccination antibody titers – Efficacy 96 -98% in prevention of cervical pre-cancers – Efficacy 30% in all vaccinated women, irrespective of baseline HPV status – Vaccine only targets 2 strains (16 and 18) so not effective in preventing genital warts – Appears to offer cross-protection against other HPV sub -types (31, 45, 52) Paavonen, Lancet 2007 and 2009

Gardasil vs Cervarix – Cervarix appears to induce higher antibody titers against HPV 16 and 18 than Gardasil – Both vaccines appear to offer cross-protection against other HPV types, but Cervarix may offer more – Gardasil also offers protection against genital warts (HPV types 6, 11) – Gardasil has demonstrated vulvar/vaginal cancer protection – Gardasil approved for use in males – Small cost difference between 2 vaccines • CDC vaccine price list- private sector cost per dose: Gardasil $130. 27 Cervarix $128. 75 Einstein et al. Hum Vaccines 2009 Paavonen et al, Lancet 2009 Medeiros et al, Int J Gynecol Cancer 2009 Bonnanni et al, Vaccine 2009

Recommendations: National Organizations • ACIP and ACOG recommend use of vaccine in females ages 9 -26 years (either quadrivalent or bivalent) • ACIP, AAFP, SAM support routine vaccination of 11 -12 year-old girls • All support catch-up vaccination for females 13 -26 yrs not previously vaccinated or who have not completed full vaccine series ACIP - Advisory Committee on Immunization Practices ACOG- American College of Obstetricians and Gynecologists AAP- American Academy of Pediatrics AAFP- American Academy of Family Physicians SAM- Society for Adolescent Medicine

Recommendations: National Organizations • Vaccine most effective if given before 1 st sexual contact • Females who have equivocal or abnormal Pap tests, positive HPV tests, or genital warts can receive HPV vaccine • Vaccine recipients should be advised that data do not indicate that the vaccine will have any therapeutic effect on existing HPV infection, cervical lesions, or genital warts • Vaccination can provide protection against infection with vaccine HPV types not already acquired

If a teen or young woman is already sexually active, or infected with HPV… No therapeutic effect demonstrated on alreadypresent HPV infection or associated disease However, vaccine still recommended – can protect against other sub-types of virus, or reinfection

HPV Vaccine • Both vaccines (HPV 4 and HPV 2) – administered as a series of 3 intramuscular injections over a 6 -month period at 0, 1 -2, and 6 months • Costs range: $120 - $150 per dose (HPV 2 may be slightly less expensive) • Covered by Vaccines for Children Program • Most insurance plans and managed care plans cover recommended vaccines • No change in Pap smear recommendations

Vaccine Scheduling Issues What happens if the teen is late for the second and/or third vaccine – do you restart the series? – Do not restart - recommendations similar to those for other childhood vaccines – Resume vaccination when teen re-presents for care

Vaccine Scheduling Issues What is the minimal interval allowable between injections – can you give the shots earlier if you worry that the teen is poorly adherent to appointment visits? – Minimal interval between injection 1 and 2 is four weeks – Minimal interval between injection 2 and 3 is 12 weeks and between 1 and 3 is 24 weeks

Safety and Side Effects of Vaccine • In clinical trials- adverse events similar in vaccine and placebo groups (HPV 4) – Headache (28%) – Dizziness (11%) – Syncope (11%) – Fever (13%) – Nausea (7%) – Injection site pain (2. 2%)- higher in injection group • Similar profile for HPV 2, more injection site symptoms FDA. gov Slade et al, JAMA 2009 Einstein et al. Hum Vaccines 2009

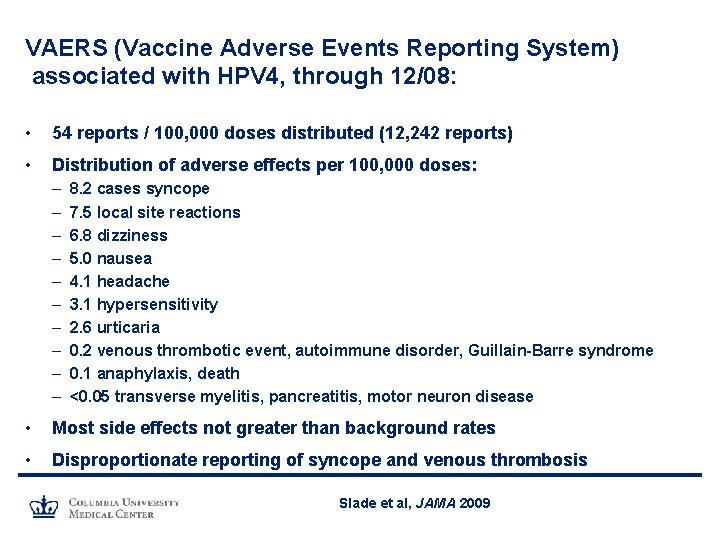
VAERS (Vaccine Adverse Events Reporting System) associated with HPV 4, through 12/08: • 54 reports / 100, 000 doses distributed (12, 242 reports) • Distribution of adverse effects per 100, 000 doses: – – – – – 8. 2 cases syncope 7. 5 local site reactions 6. 8 dizziness 5. 0 nausea 4. 1 headache 3. 1 hypersensitivity 2. 6 urticaria 0. 2 venous thrombotic event, autoimmune disorder, Guillain-Barre syndrome 0. 1 anaphylaxis, death <0. 05 transverse myelitis, pancreatitis, motor neuron disease • Most side effects not greater than background rates • Disproportionate reporting of syncope and venous thrombosis Slade et al, JAMA 2009

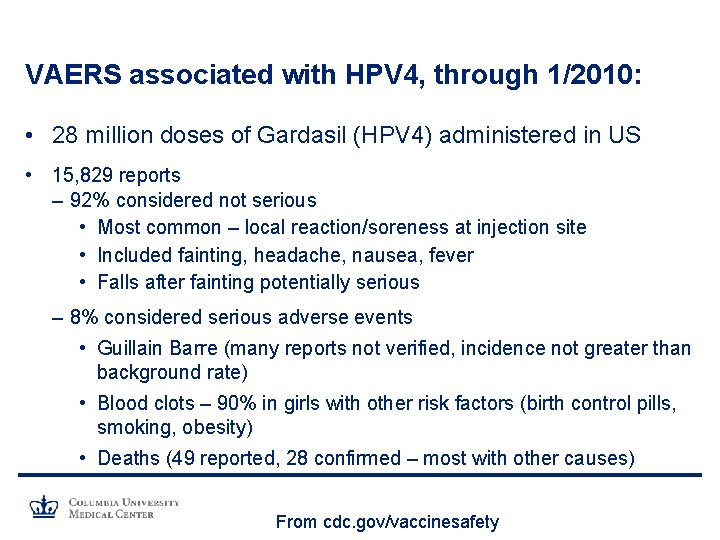
VAERS associated with HPV 4, through 1/2010: • 28 million doses of Gardasil (HPV 4) administered in US • 15, 829 reports – 92% considered not serious • Most common – local reaction/soreness at injection site • Included fainting, headache, nausea, fever • Falls after fainting potentially serious – 8% considered serious adverse events • Guillain Barre (many reports not verified, incidence not greater than background rate) • Blood clots – 90% in girls with other risk factors (birth control pills, smoking, obesity) • Deaths (49 reported, 28 confirmed – most with other causes) From cdc. gov/vaccinesafety

HPV Vaccine and Pregnancy • No studies yet on safety of vaccine during pregnancy • Currently, vaccine not recommended for pregnant women (Category B) • Those who are inadvertently vaccinated while pregnant should enroll in prenatal care and enroll in registry manufacturer is compiling to collect information on pregnancy outcomes

HPV Vaccine and Males: • In 10/09, Gardasil® (HPV 4) – FDA approved for use in males • ACIP then issued provisional permissive recommendation for vaccination of males • HPV 4 can be administered to males 9 -26 to prevent genital warts • Doctors and clinics can administer Gardasil to males at their discretion (optional vs routine vaccination) • Estimate that there are 250, 000 new cases of genital warts per year in US men

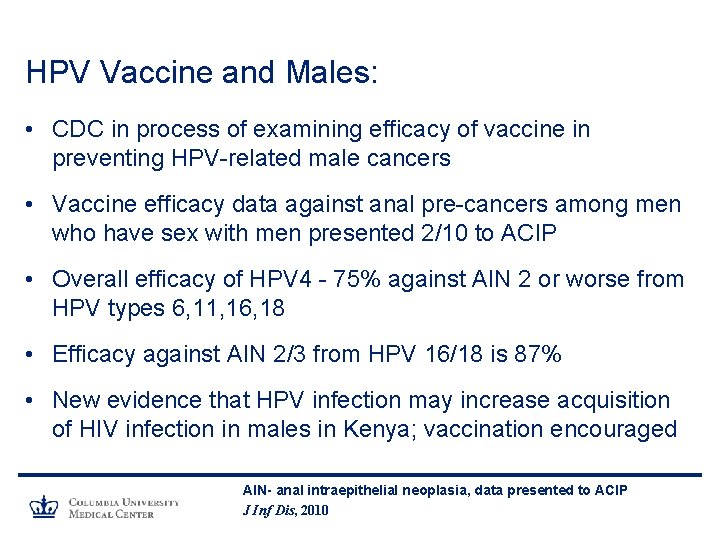
HPV Vaccine and Males: • CDC in process of examining efficacy of vaccine in preventing HPV-related male cancers • Vaccine efficacy data against anal pre-cancers among men who have sex with men presented 2/10 to ACIP • Overall efficacy of HPV 4 - 75% against AIN 2 or worse from HPV types 6, 11, 16, 18 • Efficacy against AIN 2/3 from HPV 16/18 is 87% • New evidence that HPV infection may increase acquisition of HIV infection in males in Kenya; vaccination encouraged AIN- anal intraepithelial neoplasia, data presented to ACIP J Inf Dis, 2010

HPV Vaccine and Males: • Vaccine most effective if given prior to sexual contact • Unclear if vaccination to prevent partner infection is costeffective • CDC panel recommended covering the costs of Gardasil for boys ages 9 through 18 who are beneficiaries of the federal Vaccines for Children program, which pays for vaccinations for uninsured children, those enrolled in Medicaid, or who meet other criteria

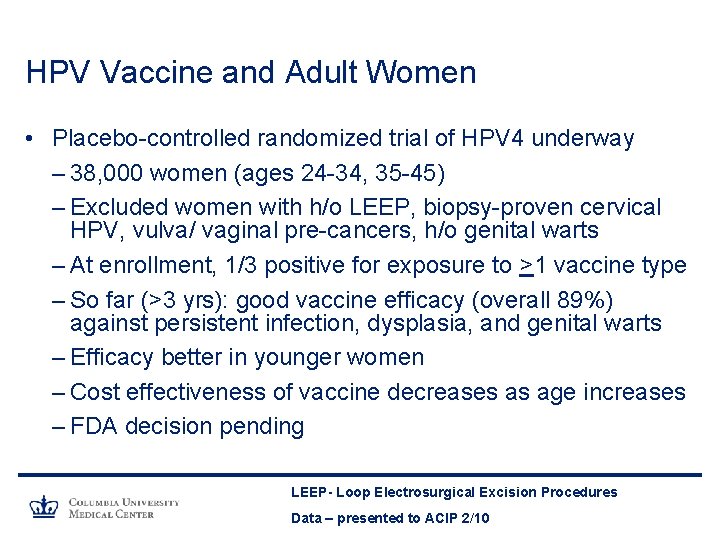
HPV Vaccine and Adult Women • Placebo-controlled randomized trial of HPV 4 underway – 38, 000 women (ages 24 -34, 35 -45) – Excluded women with h/o LEEP, biopsy-proven cervical HPV, vulva/ vaginal pre-cancers, h/o genital warts – At enrollment, 1/3 positive for exposure to >1 vaccine type – So far (>3 yrs): good vaccine efficacy (overall 89%) against persistent infection, dysplasia, and genital warts – Efficacy better in younger women – Cost effectiveness of vaccine decreases as age increases – FDA decision pending LEEP- Loop Electrosurgical Excision Procedures Data – presented to ACIP 2/10

Parental Acceptance of Vaccines • ~ 35 million US adolescents don't receive all recommended vaccines despite national recommendations • Also -unique barriers to acceptance of vaccine targeted toward preadolescents that prevents a sexually transmitted infection • HPV coverage rates: – Nationally: 37% (25% - private patients) receive 1 st vaccine – Nationally: 11 -18% patients complete series – In NYC: 48% eligible girls- receive 1 st vaccine CDC 2010 National STI Conference

Parental Acceptance • Studies demonstrate parents have high level of interest in HPV vaccine; are willing to have their children vaccinated • Important factors for parental acceptability – Vaccine efficacy – Disease severity – Physician recommendation • Physician skills in describing vaccines to adolescents/preadolescents and their parents and discussing sexuality – key for acceptance Zimet GD. J Adolesc Health. 2005; 37: S 17–S 23. Short MB et al. Curr Opin Pediatr. 2006; 18: 53– 57. Mays RM et al. Soc Sci Med. 2004; 58: 1405– 1413.

Promoting Administration of HPV Vaccine • If parent concerned about promoting risky sexual behavior… – Note that there are no data that link vaccination with earlier sexual activity – Emphasize that vaccine most effective prior to sexual activity and potential exposure to HPV – Titers appear higher if vaccinated earlier • Emphasize: – Cancer prevention, link between HPV and cervical cancer – Universal recommendations – Efficacy of the vaccine

Need for Consent • In most states, routine vaccinations can only be given to children under the age of 18 with parental consent • IN NY, HPV vaccine administration requires parental consent if given to minors, although some organizations for adolescent reproductive rights argue legal uncertainty

Should States Mandate HPV Vaccination? • 2006: Michigan senate enacted legislation to mandate vaccine for entrance to 6 th grade – but legislation not enacted • 2007: Texas governor issued order that girls be vaccinated against HPV; revoked by legislature • As of February 2010, 17 state have proposed HPVrelated legislation or resolutions • American Academy of Pediatrics not yet advocating mandatory HPV vaccination

Summary • HPV is the most common STI in adolescents and is directly linked to anogenital warts and cervical cancer • To date, HPV vaccine is safe and highly efficacious in preventing precursors to cervical cancer • Routine vaccination of 11 -12 year-old girls is supported by the CDC, ACIP and AAP, with catch-up for women through age 26 • Males can now be offered vaccination with Gardasil • Parents are generally accepting of this vaccine, especially if counseled correctly
 Karen soren
Karen soren Hpv vaccine schedule adults
Hpv vaccine schedule adults What is an alternative of log based recovery
What is an alternative of log based recovery Soren kaplan
Soren kaplan Soren prestemon
Soren prestemon Soren comp
Soren comp Soren prestemon
Soren prestemon Soren prestemon
Soren prestemon Hpv cervical cancer
Hpv cervical cancer Does hpv go away
Does hpv go away Hpv
Hpv Hpv dna testi
Hpv dna testi Meninkok
Meninkok Hpv cancer prevention
Hpv cancer prevention Epidermal dysplasia verruciformis
Epidermal dysplasia verruciformis Prevention hpv
Prevention hpv Wanzia
Wanzia Hpv type 16 and 18
Hpv type 16 and 18 Paplloma
Paplloma Hpv infektion
Hpv infektion Hsil
Hsil Muzaffer sancı
Muzaffer sancı Ahcc hpv
Ahcc hpv Hpv cervical cancer
Hpv cervical cancer Hpv type 16 and 18
Hpv type 16 and 18 Hpv
Hpv Hpv test for men
Hpv test for men Hpv
Hpv Hpv
Hpv Triage sort
Triage sort Hpv discret test
Hpv discret test Hpv us
Hpv us Kolposkopi, px
Kolposkopi, px Hpv infection
Hpv infection Genital warts
Genital warts High sierra ahec
High sierra ahec Diluent for je vaccine
Diluent for je vaccine Intervax vaccine
Intervax vaccine Vaccine cold chain monitor
Vaccine cold chain monitor Pentavalent vaccine
Pentavalent vaccine Smallpox vaccine industrial revolution description
Smallpox vaccine industrial revolution description Chickenpox vaccine schedule
Chickenpox vaccine schedule