The History of the Periodic Table Chemistry Section

The History of the Periodic Table Chemistry Section 5 -1

Targets for Today Describe the concept of alchemy Describe early scientific breakthroughs in the discovery of elements Explain how the modern periodic table is constructed.

The Discovery of Elements

The world is made out of stuff People have known and named specific elements since ancient times Ex. Gold, silver, tin, copper, lead, mercury

I discovered something, but what is it? Early alchemists isolated some elements from compounds, though they didn’t know they had the first pure element. Alchemy – a scientists whose goal is to turn basic metals (ex. Copper) into gold

Arsenic One of the earliest elements Arsenic (#33) is an extremely toxic element, and after it was discovered it’s primary use became murder.

Arsenic Known in Roman times and used to poison rivals and even emperors. White arsenic, which is arsenic oxide, is a water-soluble, tasteless solid easily added to drinks. This material was obtained as a by-product of copper and lead refining. It the 1600 s it was sold by agents of a woman known as Toffana of Sicily, to people who wished to dispose of someone and it became known as “inheritance powder”. In the 1800 s arsenic compounds became widely available – as weed -killers, flypapers, rat poisons, etc. – and were used in domestic murders, being cited in many famous murder cases.

Arsenic Symptoms Headaches, confusion, severe diarrhea, and drowsiness. As the poisoning develops, convulsions and changes in fingernail pigmentation may occur. When the poisoning becomes acute, symptoms may include diarrhea, vomiting, blood in the urine, cramping muscles, hair loss, stomach pain, and more convulsions. The organs of the body that are usually affected by arsenic poisoning are the lungs, skin, kidneys, and liver. The final result of arsenic poisoning is coma to death

Getting away with murder Arsenic was undetectable as a murder weapon since its discovery in 1250, until the chemist James Marsh invented the “Marsh Test” in 1836 for use in forensic toxicology.

Forensics Def: The use of science to answer questions of interest to the legal system.

Looking for a career? Forensic Pathologist A doctor who focuses on the medico-legal investigation of sudden or unexpected death. These are the guys who perform autopsies Salary: ranges from $60, 000 -180, 000 depending on the size of the city

What to do now? These are the recommendations from a forensic pathologist to high school students: Take four years of English, science, and math Take as many high-level courses as you can handle (to test out of basic college classes) Be able to write a clear, grammatically correct report by the time you graduate Get involved in speech/debate team


The first true element - Phosphorus In 1669 an alchemist in Hamburg, Germany named Hennig Brand was the first person to discover an element and know what it was.

Hennig Brand He discovered phosphorus after isolating it from urine… over 1, 200 gallons of it! As he boiled it down in an attempt to make a “sorcerer’s stone, ” a yellowish-green powder remained

Phosphorus Characteristics Atomic # 15 White powder that emits a faint glow when exposed to oxygen Only water and calcium make up more of your bodies weight

John Davy (1788 -1829) Davy discovered a love of chemistry at age 19, and became one of the most celebrated chemists of his day. He discovered a number of elements, including: calcium, sodium, potassium, magnesium, and others.


John Davy (1788 -1829) Other discoveries Nitrous oxide (laughing gas) Inventions One of the first light bulbs Miner headlamps

Radioactive Elements In 1891 the first radioactive elements was discovered – Uranium (#92) Its unusual properties were not discovered until much later.

The Noble Gases In 1868 a new gas (helium) was discovered, which had been previously overlooked because it was inert. Soon other similar gases were discovered, and they nonreactive gases were given the name noble gases. An inert chemical is one that is stable and unreactive under specified conditions. At one time, the noble gases in Group 18 of the periodic table were known as the inert gases, because they had not been observed to form any compounds.

The Making of the Periodic Table

The First Periodic Table Alexandre de Chancourtois (a French geologist) was the first to produce a periodic table with elements listed based on atomic mass (1862) His periodic table contained all of the known elements at the time – 53 in all.


The Modern Periodic Table Dmitri Mendeleev, a Russian chemist and inventor, published the periodic table in the form we use today.
Mendeleev His periodic table was also based on atomic mass, though he had the foresight to leave empty spaces on the table for the elements he knew would be discovered in the future.
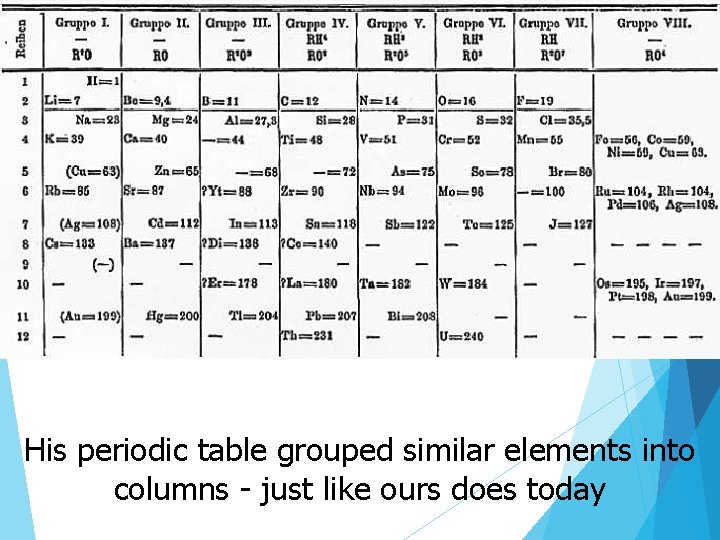
His periodic table grouped similar elements into columns - just like ours does today
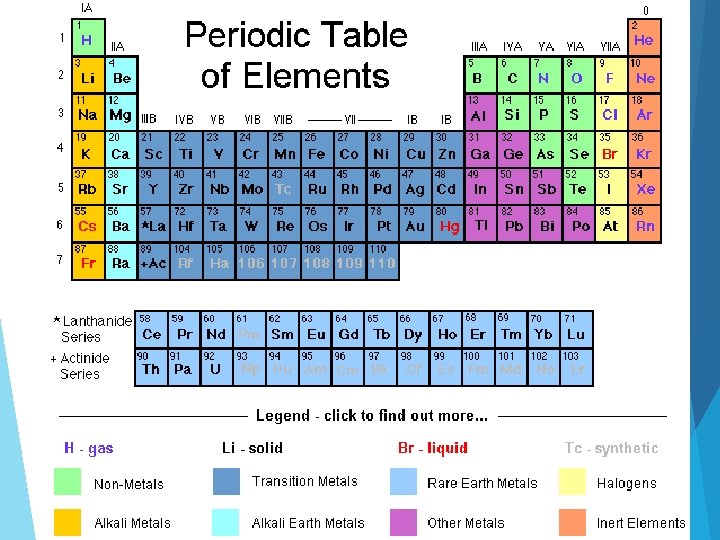
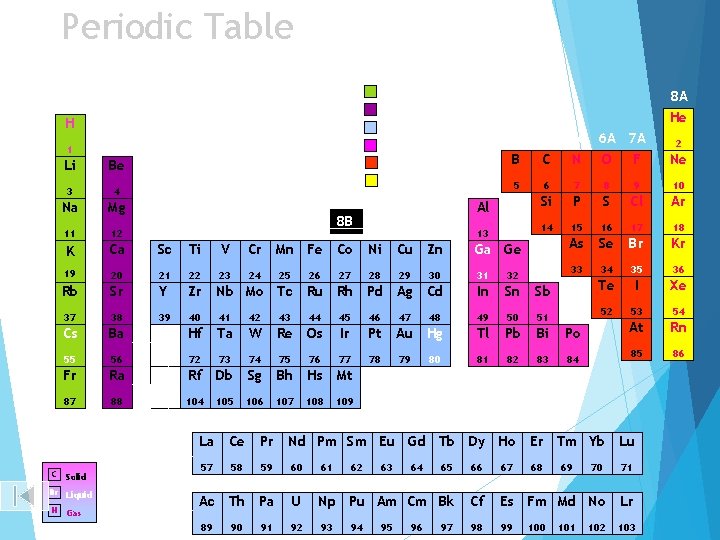
Periodic Table Alkali earth metals H 1 2 3 4 5 6 7 8 A He Alkali metals 1 A Transition metals 1 2 A Boron group Li Be Nonmetals 3 4 Na Mg 11 12 K 3 A 4 A B C Noble gases Ca 8 B 3 B 4 B 5 B 6 B 7 B Sc Ti V Cr Mn Fe Co 19 20 21 22 23 Rb Sr Y 37 38 39 Cs 5 Al 5 A 6 A 7 A N O F 7 8 9 10 Si P S Cl Ar 14 15 16 17 18 As Se Br Kr 33 34 35 36 Te I Xe 52 53 54 At Rn 85 86 Ni Ga Ge 28 29 30 31 32 Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb 40 41 42 43 44 45 46 47 48 49 50 51 Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po 55 56 72 73 74 75 76 77 78 79 81 82 83 84 Fr Ra Rf Db Sg Bh Hs Mt 87 88 104 105 106 107 109 24 25 26 27 108 80 Lanthanoid Series 6 C Solid Br Liquid H Gas La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 58 59 60 61 65 66 67 68 70 71 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr 89 92 93 98 99 103 62 63 64 69 Actinoid Series 7 90 91 94 95 96 97 Ne 6 1 B 2 B Cu Zn 13 2 100 101 102
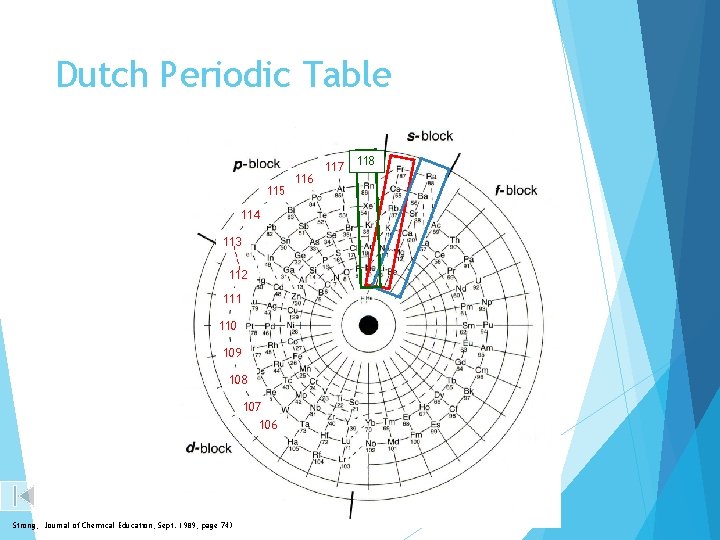
Dutch Periodic Table 115 114 113 112 111 110 109 108 107 106 Strong, Journal of Chemical Education, Sept. 1989, page 743 116 117 118
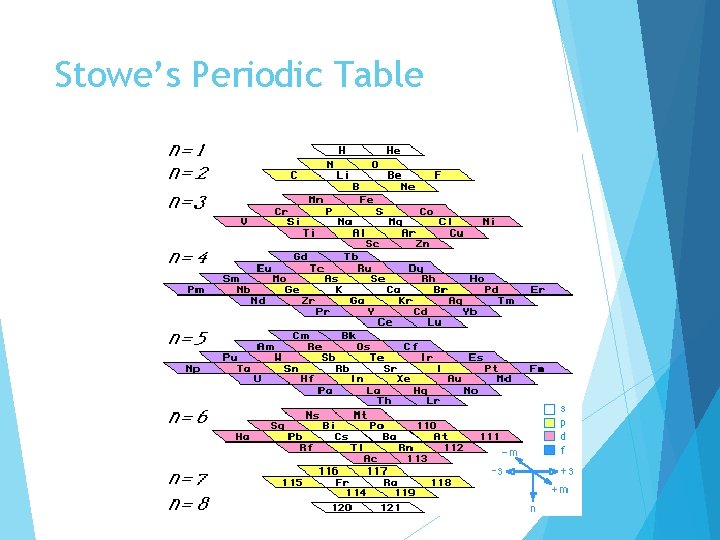
Stowe’s Periodic Table
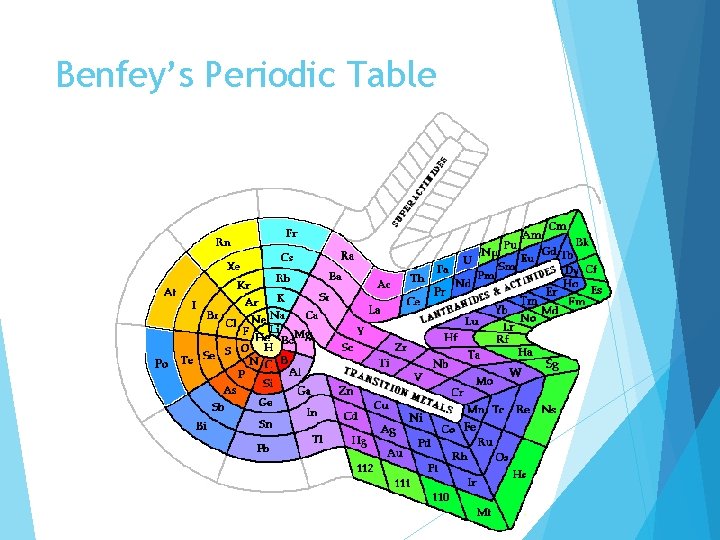
Benfey’s Periodic Table

Döbereiner’s Triads Johann Döbereiner ~1817 Name Atomic Mass Calcium Barium 40 137 Chlorine Iodine 35. 5 127 Sulfur Tellurium 32 127. 5 Average 88. 5 Average 81. 3 Average 79. 8 Bromine 79. 9 Selenium 79. 2 Strontium 87. 6 Döbereiner discovered groups of three related elements which he termed a triad. Smoot, Price, Smith, Chemistry A Modern Course 1987, page 161
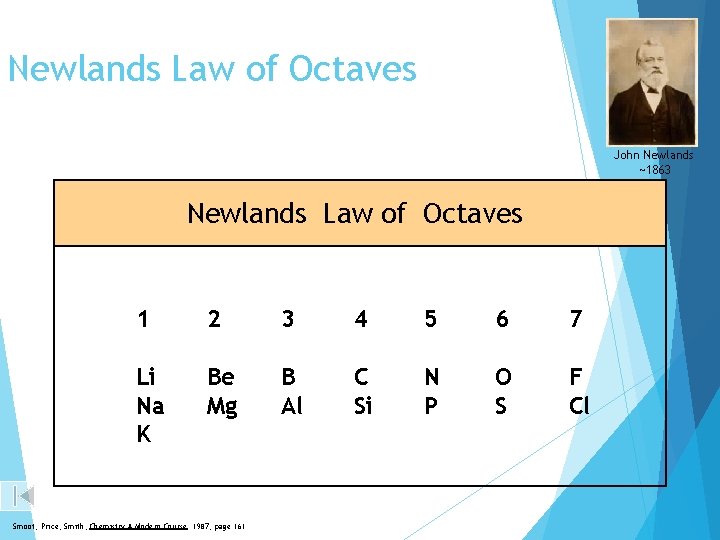
Newlands Law of Octaves John Newlands ~1863 Newlands Law of Octaves 1 2 3 4 5 6 7 Li Na K Be Mg B Al C Si N P O S F Cl Smoot, Price, Smith, Chemistry A Modern Course 1987, page 161
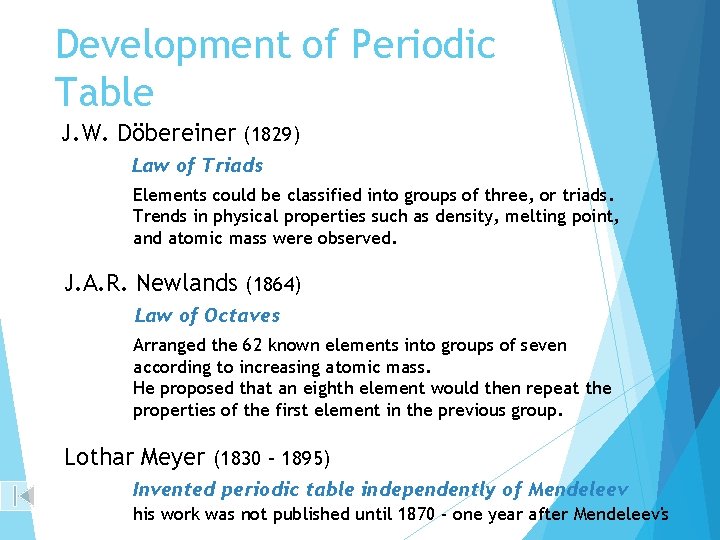
Development of Periodic Table J. W. Döbereiner (1829) Law of Triads Elements could be classified into groups of three, or triads. Trends in physical properties such as density, melting point, and atomic mass were observed. J. A. R. Newlands (1864) Law of Octaves Arranged the 62 known elements into groups of seven according to increasing atomic mass. He proposed that an eighth element would then repeat the properties of the first element in the previous group. Lothar Meyer (1830 – 1895) Invented periodic table independently of Mendeleev his work was not published until 1870 - one year after Mendeleev's

Dmitri Mendeleev Russian Invented periodic table Organized elements by properties Arranged elements by atomic mass Predicted existence of several unknown elements Element 101 Dmitri Mendeleev

Dmitri Mendeléev
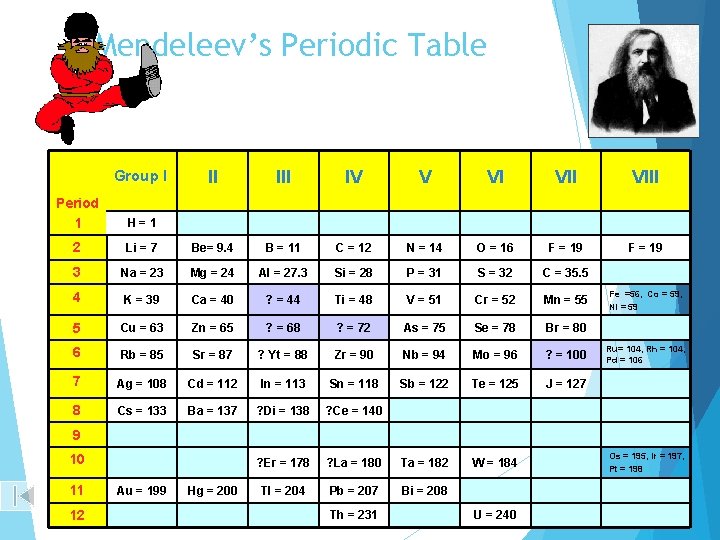
Mendeleev’s Periodic Table Group I II IV V VI VIII F = 19 Period 1 H=1 2 Li = 7 Be= 9. 4 B = 11 C = 12 N = 14 O = 16 F = 19 3 Na = 23 Mg = 24 Al = 27. 3 Si = 28 P = 31 S = 32 C = 35. 5 4 K = 39 Ca = 40 ? = 44 Ti = 48 V = 51 Cr = 52 Mn = 55 5 Cu = 63 Zn = 65 ? = 68 ? = 72 As = 75 Se = 78 Br = 80 6 Rb = 85 Sr = 87 ? Yt = 88 Zr = 90 Nb = 94 Mo = 96 ? = 100 7 Ag = 108 Cd = 112 In = 113 Sn = 118 Sb = 122 Te = 125 J = 127 8 Cs = 133 Ba = 137 ? Di = 138 ? Ce = 140 ? Er = 178 ? La = 180 Ta = 182 W = 184 Tl = 204 Pb = 207 Bi = 208 Fe =56, Co = 59, Ni = 59 Ru= 104, Rh = 104, Pd = 106 9 10 11 12 Au = 199 Hg = 200 Th = 231 U = 240 Os = 195, Ir = 197, Pt = 198
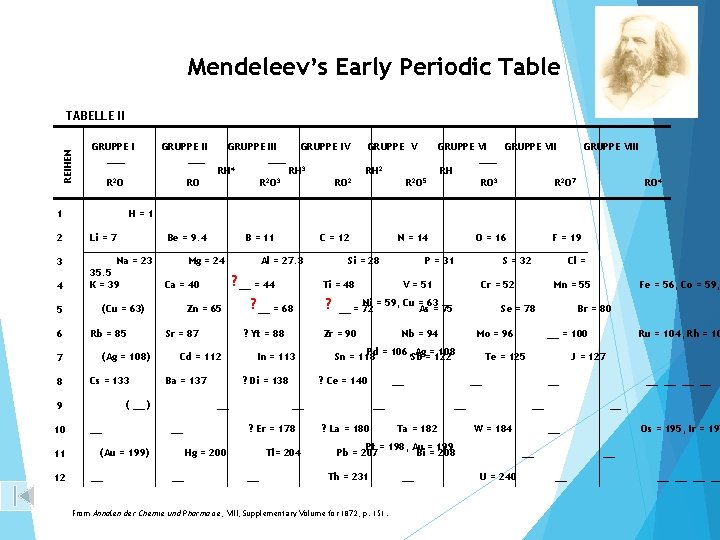
Mendeleev’s Early Periodic Table REIHEN TABELLE II GRUPPE I ___ R 2 O RO 1 Li = 7 3 4 Na = 23 35. 5 K = 39 5 (Cu = 63) (Ag = 108) Cs = 133 11 12 __ GRUPPE V RH 2 R 2 O 5 Zn = 65 Sr = 87 Al = 27. 3 GRUPPE VIII ___ RH RO 3 R 2 O 7 RO 4 Ba = 137 ? __ = 68 ? Di = 138 __ Si = 28 ? Tl= 204 __ Mo = 96 Ta = 182 From Annalen der Chemie und Pharmacie, Pharmacie VIII, Supplementary Volume for 1872, p. 151. __ __ = 100 __ __ W = 184 __ __ U = 240 Ru = 104, Rh = 10 J = 127 __ __ Fe = 56, Co = 59, Br = 80 Te = 125 Pt = 198, Au = 199 Pb = 207 Bi = 208 Th = 231 Mn = 55 Se = 78 __ ? La = 180 Cl = Cr = 52 Nb = 94 __ F = 19 S = 32 Pd = 106, Sb Ag==122 108 Sn = 118 __ Hg = 200 P = 31 Ni = 59, Cu = 63 __ = 72 As = 75 ? Ce = 140 ? Er = 178 O = 16 V = 51 Zr = 90 In = 113 __ N = 14 Ti = 48 ? Yt = 88 Cd = 112 __ C = 12 ? __ = 44 Ca = 40 (Au = 199) __ B = 11 Mg = 24 ( __ ) 9 10 Be = 9. 4 Rb = 85 7 8 GRUPPE III GRUPPE IV ___ RH 4 RH 3 2 3 RO RO 2 H=1 2 6 GRUPPE II ___ Os = 195, Ir = 197 __ __ __
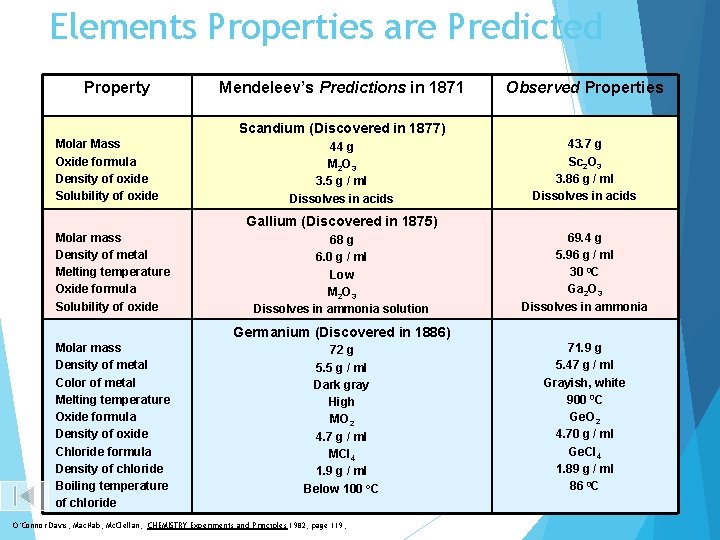
Elements Properties are Predicted Property Mendeleev’s Predictions in 1871 Observed Properties Scandium (Discovered in 1877) Molar Mass Oxide formula Density of oxide Solubility of oxide 44 g M 2 O 3 3. 5 g / ml Dissolves in acids 43. 7 g Sc 2 O 3 3. 86 g / ml Dissolves in acids Gallium (Discovered in 1875) Molar mass Density of metal Melting temperature Oxide formula Solubility of oxide 68 g 6. 0 g / ml Low M 2 O 3 Dissolves in ammonia solution 69. 4 g 5. 96 g / ml 30 0 C Ga 2 O 3 Dissolves in ammonia Germanium (Discovered in 1886) Molar mass Density of metal Color of metal Melting temperature Oxide formula Density of oxide Chloride formula Density of chloride Boiling temperature of chloride 72 g 5. 5 g / ml Dark gray High MO 2 4. 7 g / ml MCl 4 1. 9 g / ml Below 100 o. C O’Connor Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 119, 71. 9 g 5. 47 g / ml Grayish, white 900 0 C Ge. O 2 4. 70 g / ml Ge. Cl 4 1. 89 g / ml 86 0 C
Periodic Table of the Elements 1 2 3 4 5 6 7 H He 1 2 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Cu Zn Ga Ge As Se Br Kr Na Mg 11 12 K Ca Sc Ti V Cr Mn Fe Co Ni 19 20 21 22 23 24 28 Rb Sr Y Zr 37 38 39 40 41 42 Cs Ba Hf Ta W 55 56 72 73 74 Fr Ra Rf Db 87 88 104 * W 25 26 27 29 30 Nb Mo Tc Ru Rh Pd Ag Cd 34 35 36 In Sn Sb Te I Xe 49 50 51 52 53 54 Tl Pb Bi Po At Rn 81 82 83 84 85 86 46 Re Os Ir Pt 76 77 78 Sg Bh Hs Mt 105 106 107 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho 57 58 59 60 Ac Th Pa U 89 92 91 108 61 79 Er Tm Yb Lu 67 Np Pu Am Cm Bk Cf Es Fm Md No Lr 98 99 103 95 64 80 66 94 63 Au Hg 65 93 62 48 33 45 75 47 32 44 90 43 31 96 97 68 100 69 101 70 102 71

Modern Periodic Table Henry G. J. Moseley Determined the atomic numbers of elements from their X-ray spectra (1914) Arranged elements by increasing atomic number Killed in WW I at age 28 (Battle of Gallipoli in Turkey) 1887 - 1915
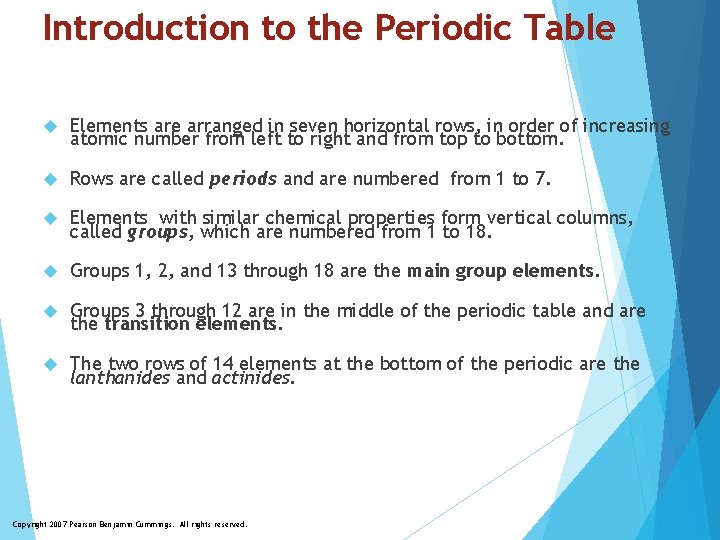
Introduction to the Periodic Table Elements are arranged in seven horizontal rows, in order of increasing atomic number from left to right and from top to bottom. Rows are called periods and are numbered from 1 to 7. Elements with similar chemical properties form vertical columns, called groups, which are numbered from 1 to 18. Groups 1, 2, and 13 through 18 are the main group elements. Groups 3 through 12 are in the middle of the periodic table and are the transition elements. The two rows of 14 elements at the bottom of the periodic are the lanthanides and actinides. Copyright 2007 Pearson Benjamin Cummings. All rights reserved.
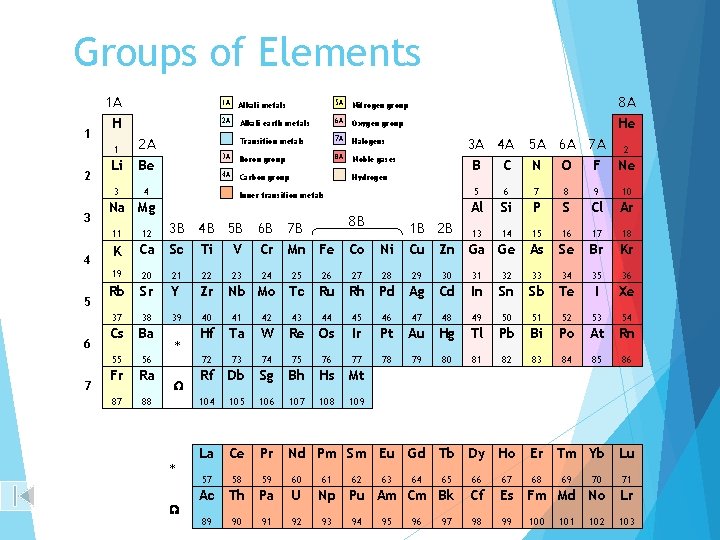
Groups of Elements 1 A 1 2 3 4 5 6 7 H 1 2 A Li Be 3 4 1 A Alkali metals 5 A Nitrogen group 2 A Alkali earth metals 6 A Oxygen group Transition metals 7 A Halogens 3 A Boron group 8 A Noble gases 4 A Carbon group 8 A He Hydrogen Inner transition metals Na Mg 8 B 11 12 3 B K Ca Sc 4 B 5 B Ti V 19 20 21 22 Rb Sr Y Zr 37 38 39 40 41 42 Cs Ba Hf Ta W 55 56 72 73 74 Fr Ra Rf Db 87 88 104 * W 23 6 B 7 B Cr Mn Fe Co 24 25 26 27 Ni 28 4 A C 5 A N 6 A O 5 6 7 8 9 10 Al Si P S Cl Ar 16 17 18 Se Br Kr 1 B 2 B 13 14 15 Cu Zn Ga Ge As 29 30 Nb Mo Tc Ru Rh Pd Ag Cd 33 34 35 36 In Sn Sb Te I Xe 49 50 51 52 53 54 Tl Pb Bi Po At Rn 81 82 83 84 85 86 46 Re Os Ir Pt 76 77 78 Sg Bh Hs Mt 105 106 107 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho 57 58 59 60 Ac Th Pa U 89 92 91 108 61 79 Er Tm Yb Lu 67 Np Pu Am Cm Bk Cf Es Fm Md No Lr 98 99 103 95 64 80 66 94 63 Au Hg 65 93 62 48 32 45 75 47 7 A 2 F Ne 31 44 90 43 3 A B 96 97 68 100 69 101 70 102 71
Alkali Metals, Group 1 H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

Alkaline Earth Metals, Group 2 H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Halogens, Group 17 H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

Noble Gases, Group 18 H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Chalcogens/Oxygen, Group 16 H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Pnicogens/Nitrogen, Group 15 H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

Chemistry of the Groups Group 15, the Pnicogens – The pnicogens are nitrogen, phosphorus, arsenic, antimony, and bismuth. 15 N 7 P 15 As – All the pnicogens have ns 2 np 3 valence-electron configurations, leading to three common oxidation states: 1. – 3, in which three electrons are added to give the closed-shell electron configuration of the next noble gas 2. +5, in which all five valence electrons are lost to give the closed-shell electron configuration of the preceding noble 3. +3, in which only the three np electrons are lost to give a 2 Sb filled ns subshell 33 51 Bi 83 Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. gas

Chemistry of the Groups Group 14 – Group 14 elements straddle the diagonal line that divides nonmetals from metals. – Carbon is a nonmetal, silicon and germanium are semimetals, and tin and lead are metals. – Group-14 elements have the ns 2 np 2 valence-electron configuration. – Group-14 elements have three oxidation states: 1. – 4, in which four electrons are added to achieve the closed-shell electron configuration of the next noble gas 2. +4, in which all four valence electrons are lost to give the closedelectron configuration of the preceding noble gas 3. +2, in which the loss of two np 2 electrons gives a filled ns 2 subshell Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. shell
Chemistry of the Groups Group 13 – Of the Group-13 elements, only the lightest, boron, lies on the diagonal line that separates nonmetals and metals, it is a semimetal and possesses an unusual structure. – The rest of Group 13 are metals (aluminum, gallium, indium, and thallium) and are typical metallic solids. – Elements of Group 13 are highly reactive and form stable compounds with oxygen. – Elements of Group 13 have ns 2 np 1 valence-electron configurations. Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Lanthanide Series/ Rare Earth Metals H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Actinide Series H He F Ne Li Be B C N O Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Chemistry of the Groups 1 A 1 2 3 4 5 6 7 8 A He H 1 2 A Li Be 3 4 Transition Metals Na Mg 8 B 11 12 3 B K Ca Sc 4 B 5 B Ti V 19 20 21 22 Rb Sr Y Zr 37 38 39 40 41 42 Cs Ba Hf Ta W 55 56 72 73 74 Fr Ra Rf Db 87 88 104 * W Lanthanides * Actinides W 23 6 B 7 B Cr Mn Fe Co 24 25 26 27 Ni 28 4 A C 5 A N 6 A O 5 6 7 8 9 10 Al Si P S Cl Ar 16 17 18 Se Br Kr 1 B 2 B 13 14 15 Cu Zn Ga Ge As 29 30 Nb Mo Tc Ru Rh Pd Ag Cd 33 34 35 36 In Sn Sb Te I Xe 49 50 51 52 53 54 Tl Pb Bi Po At Rn 81 82 83 84 85 86 46 Re Os Ir Pt 76 77 78 Sg Bh Hs Mt 105 106 107 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho 57 58 59 60 Ac Th Pa U 89 92 91 108 61 79 Er Tm Yb Lu 67 Np Pu Am Cm Bk Cf Es Fm Md No Lr 98 99 103 95 64 80 66 94 63 Au Hg 65 93 62 48 32 45 75 47 7 A 2 F Ne 31 44 90 43 3 A B 96 97 68 100 69 101 70 102 71
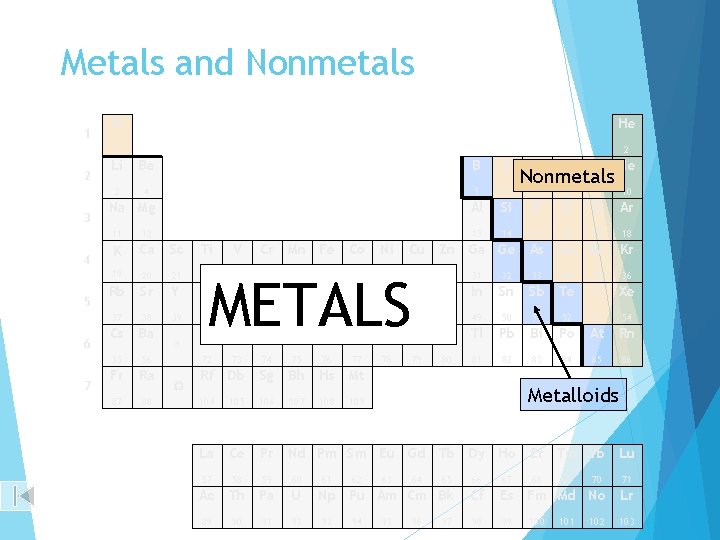
Metals and Nonmetals 1 2 3 4 5 6 7 H He 1 2 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Cu Zn Ga Ge As Se Br Kr Na Mg 11 12 K Ca Sc Ti 19 20 21 22 Rb Sr Y Zr 37 38 39 40 41 42 Cs Ba Hf Ta W 55 56 72 73 74 Fr Ra Rf Db 87 88 104 * W V Cr Mn Fe Co Ni METALS 23 24 25 26 27 28 29 30 Nb Mo Tc Ru Rh Pd Ag Cd 33 34 35 36 In Sn Sb Te I Xe 49 50 51 52 53 54 Tl Pb Bi Po At Rn 81 82 83 84 85 86 46 Re Os Ir Pt 76 77 78 Sg Bh Hs Mt 105 106 107 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho 57 58 59 60 Ac Th Pa U 89 92 91 108 61 Au Hg 79 80 Metalloids 66 67 Np Pu Am Cm Bk Cf Es Fm Md No Lr 98 99 103 94 63 95 64 Er Tm Yb Lu 65 93 62 48 32 45 75 47 31 44 90 43 Nonmetals 96 97 68 100 69 101 70 102 71

Metals, Nonmetals, & Metalloids 1 Nonmetals 2 3 4 5 Metals 6 7 Metalloids Zumdahl, De. Coste, World of Chemistry 2002, page 349
Properties of Metals, Nonmetals, and Metalloids METALS malleable, lustrous, ductile, good conductors of heat and electricity NONMETALS gases or brittle solids at room temperature, poor conductors of heat and electricity (insulators) METALLOIDS (Semi-metals) dull, brittle, semi-conductors (used in computer chips)
- Slides: 60