The History of the Atom Learning Objectives TBAT
The History of the Atom Learning Objectives: TBAT use the evidence from Geiger and Marsden to explain the structure of an atom. TBAT describe how the model of the atom has changed. TBAT describe the model of the atom. 1 of 40 © Boardworks Ltd 2007
What are atoms? It is now known that all matter is made of atoms. In some substances, all the atoms are the same, which means that the substance is called an element. For example, gold is an element made up of only gold atoms. It is only relatively recently that we have had microscopes powerful enough to ‘see’ individual atoms. Before that, the idea that atoms existed was only a theory. The first person to suggest the idea of atoms was the Greek philosopher Democritus, in 450 BC. 2 of 40 © Boardworks Ltd 2007

What did Dalton think atoms were like? Ideas about atomic structure have changed over time. In 1803, John Dalton reintroduced the idea that everything is made of atoms. He said atoms were solid spheres of matter that could not be split. Dalton also suggested that each element contained identical atoms. 3 of 40 © Boardworks Ltd 2007

How did electrons spoil Dalton’s model? In 1897, whilst studying cathode rays, JJ Thomson discovered tiny particles with a negative charge. These negative particles were given out by atoms and were much smaller than atoms. Thomson had discovered the existence of electrons. His discovery did not fit with Dalton’s model of the atom, and so Thomson had to propose a new model. 4 of 40 © Boardworks Ltd 2007
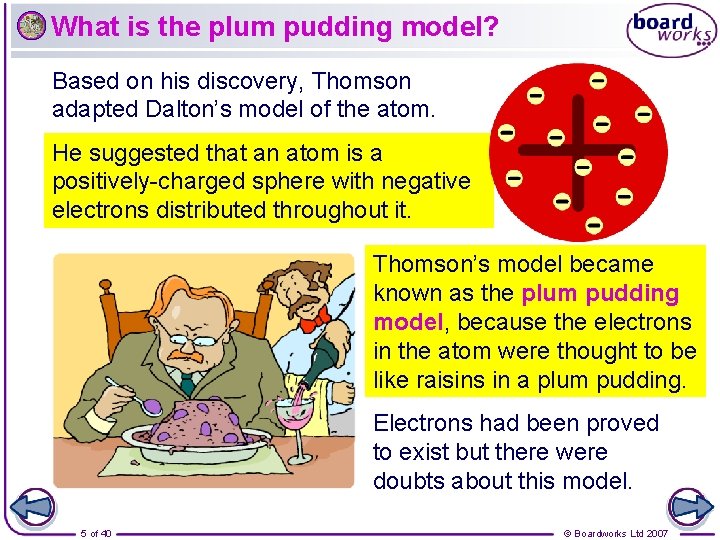
What is the plum pudding model? Based on his discovery, Thomson adapted Dalton’s model of the atom. He suggested that an atom is a positively-charged sphere with negative electrons distributed throughout it. Thomson’s model became known as the plum pudding model, because the electrons in the atom were thought to be like raisins in a plum pudding. Electrons had been proved to exist but there were doubts about this model. 5 of 40 © Boardworks Ltd 2007
What was Rutherford’s involvement? 6 of 40 © Boardworks Ltd 2007
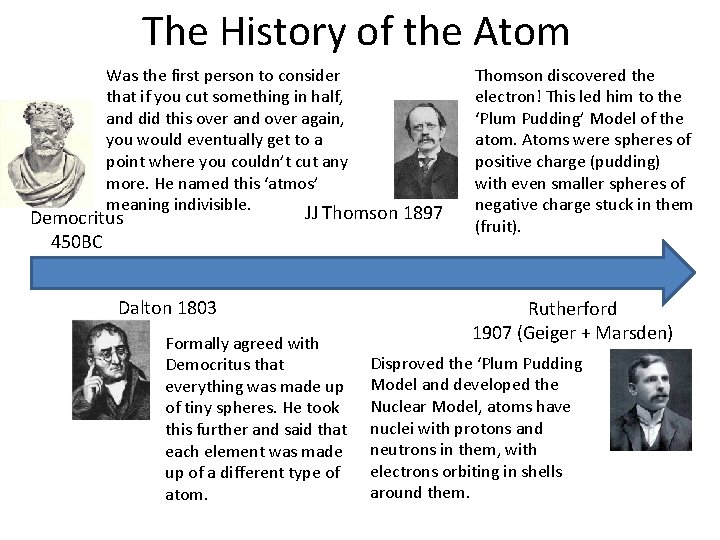
The History of the Atom Was the first person to consider that if you cut something in half, and did this over and over again, you would eventually get to a point where you couldn’t cut any more. He named this ‘atmos’ meaning indivisible. JJ Thomson 1897 Democritus 450 BC Dalton 1803 Formally agreed with Democritus that everything was made up of tiny spheres. He took this further and said that each element was made up of a different type of atom. Thomson discovered the electron! This led him to the ‘Plum Pudding’ Model of the atom. Atoms were spheres of positive charge (pudding) with even smaller spheres of negative charge stuck in them (fruit). Rutherford 1907 (Geiger + Marsden) Disproved the ‘Plum Pudding Model and developed the Nuclear Model, atoms have nuclei with protons and neutrons in them, with electrons orbiting in shells around them.

What did Geiger and Marsden do? 8 of 40 © Boardworks Ltd 2007

What were Geiger and Marsden’s results? The results of Geiger and Marsden’s experiment were: 2. Some alpha particles were slightly deflected by the gold foil. 3. A few alpha particles were bounced back from the gold foil. 1. Most alpha particles went straight through the gold foil, without any deflection. The experiment was carried out in a vacuum, so deflection of the alpha particles must have been due to the gold foil. How can these results be explained in terms of atoms? 9 of 40 © Boardworks Ltd 2007
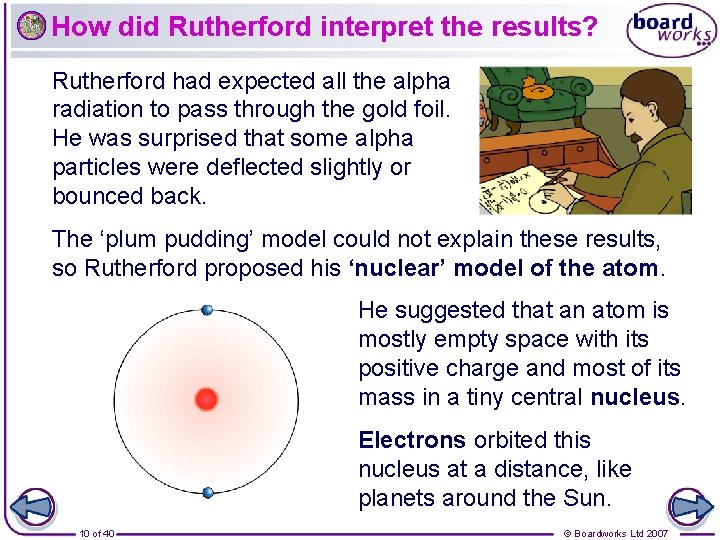
How did Rutherford interpret the results? Rutherford had expected all the alpha radiation to pass through the gold foil. He was surprised that some alpha particles were deflected slightly or bounced back. The ‘plum pudding’ model could not explain these results, so Rutherford proposed his ‘nuclear’ model of the atom. He suggested that an atom is mostly empty space with its positive charge and most of its mass in a tiny central nucleus. Electrons orbited this nucleus at a distance, like planets around the Sun. 10 of 40 © Boardworks Ltd 2007
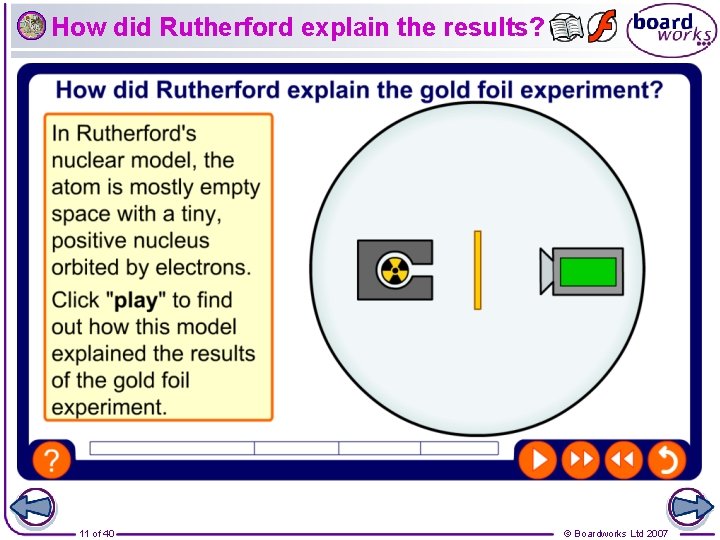
How did Rutherford explain the results? 11 of 40 © Boardworks Ltd 2007
Which model of the atom? 12 of 40 © Boardworks Ltd 2007

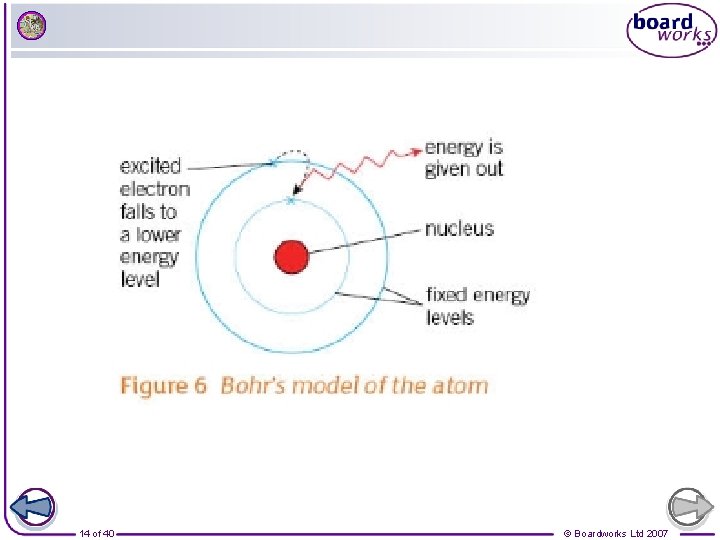
Evidence for electrons in shells (energy levels) Ø The next important development came in 1914 when Niels Bohr revised the atomic model again. Ø He noticed that the light given out when atoms were heated only had specific amounts of energy Ø He concluded that the electrons must be orbiting the nucleus at set distances in certain fixed energy levels (or shells) Ø The energy must be given out when `excited electrons fall from a high to a low energy level 13 of 40 © Boardworks Ltd 2007
14 of 40 © Boardworks Ltd 2007

Evidence for neutrons in the nucleus In 1932 James Chadwick found two types of sub -atomic particles inside the nucleus. He had evidence for the existence of protons but then realised that the other sub-atomic particle was the neutron as it had no charge and had a mass of 1 amu 15 of 40 © Boardworks Ltd 2007
- Slides: 15