The History of the Atom Discovery of Small










- Slides: 33

The History of the Atom

Discovery of Small Particles • The Greeks • John Dalton

Democritus 460 BC - 370 BC • Coined the term ‘atomos’ • All matter is composed of small, indivisible particles (atoms) • No experimentation

Aristotle emphasized that nature consisted of four elements: air, earth, fire, and water.

John Dalton’s Solid Sphere Model • In 1803, John Dalton was the first to show laboratory evidence of the existence of atoms. • Dalton’s model of the atom was a solid sphere with no subatomic particles…the atom was indivisible! • Dalton also proposed the first atomic theory which stated that all things are made up of atoms and these atoms can combine in simple whole number ratios to form compounds.

John Dalton's Atomic Theory 1) All matter is made of atoms. Atoms are indivisible and indestructible. 2) All atoms of a given element are identical in mass and properties 3) Compounds are formed by a combination of two or more different kinds of atoms. 4) A chemical reaction is a rearrangement of atoms.

Problems with Dalton’s Atomic Theory? 1. Matter is composed of small indivisible particles Atoms Can Be Divided, but only in a nuclear reaction 2. All atoms of a particular element are identical Does Not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. Different elements have different atoms YES! 4. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements. Yes, except for nuclear reactions that can change atoms of one element to a different element

Discovery of the Electron • William Crookes • J. J Thomson • Robert Millikan

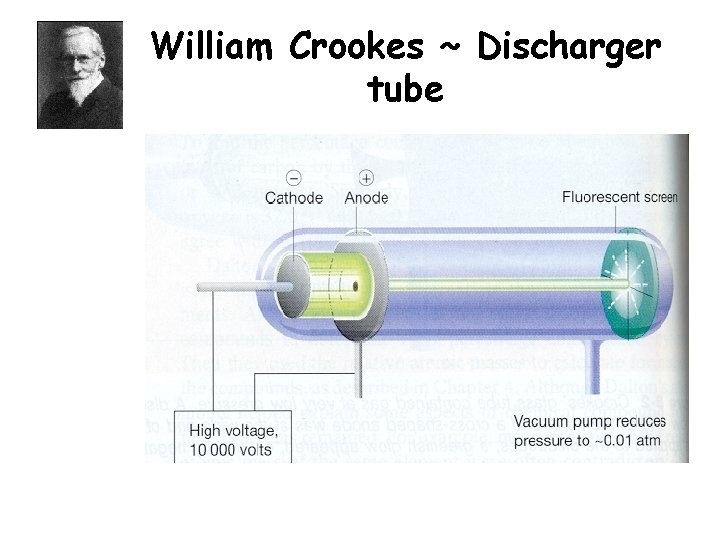
William Crookes ~ Discharger tube

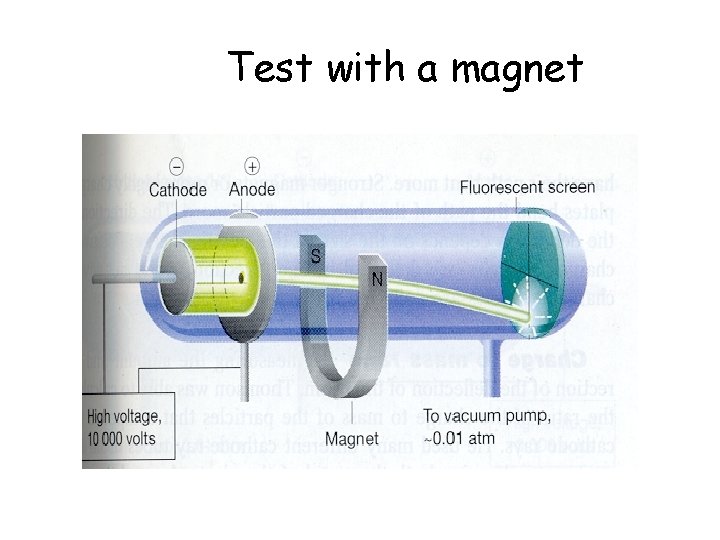
Test with a magnet

The paddle wheel experiment

J. J. Thomson: Cathode Rays Cathode rays can be deflected by a magnetic field.

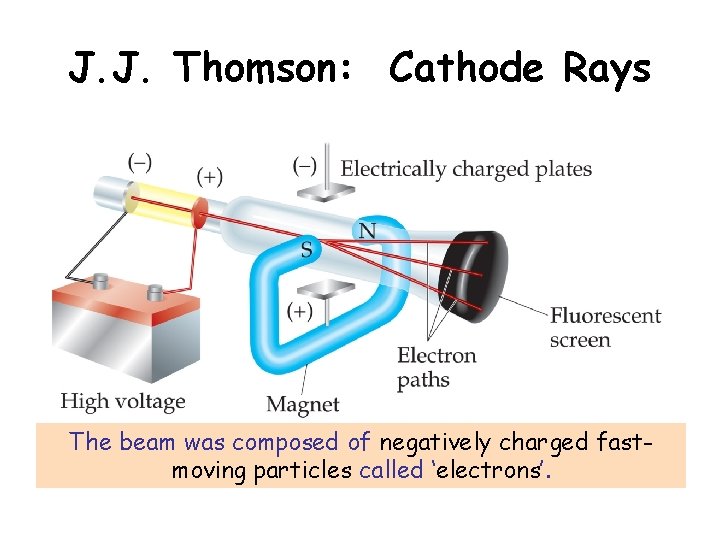
J. J. Thomson: Cathode Rays The beam was composed of negatively charged fastmoving particles called ‘electrons’.

What were these particles? ? ? • Thomson reasoned that since the beam came from the cathode (a negative electrode), the particles may have a negative charge. • Studied the deflection of the cathode ray by an electric field to determine if this was the case • By studying factors that effected the deflection of the beam, Thomson calculated the charge to mass ratio of the par

Robert A. Millikan Established the charge of an electron and Determined the atomic structure of electricity

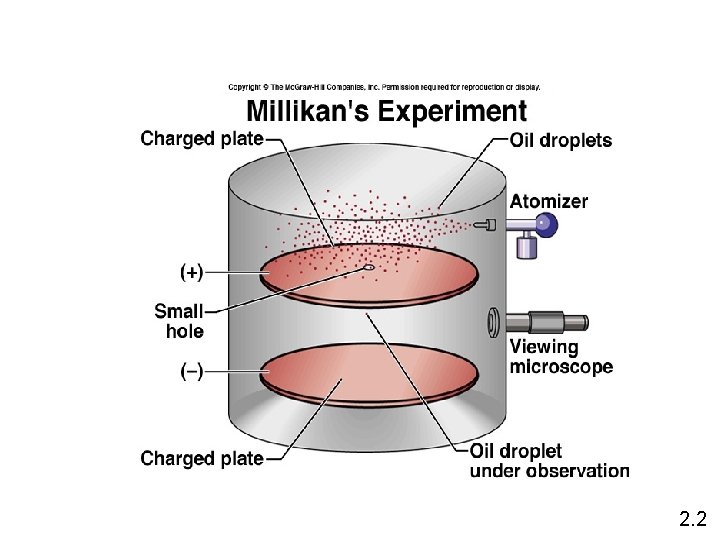
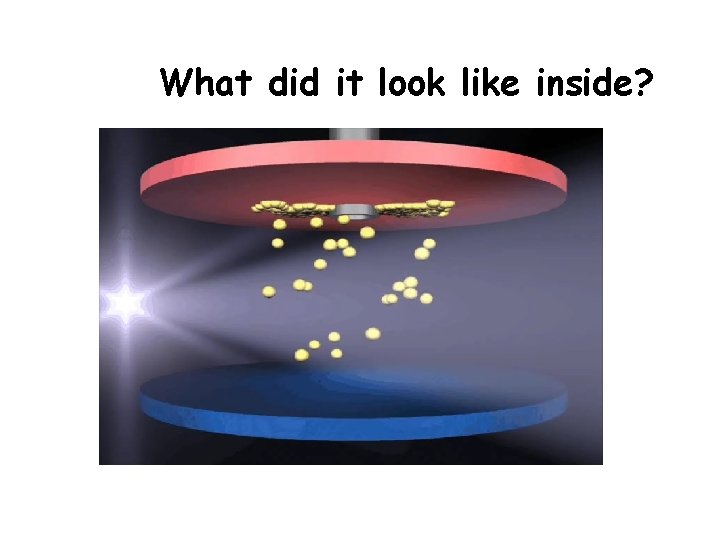
Millikan’s Oil Drop Experiment • When he sprayed oil droplets into a chamber and bombarded them with X-rays to place a negative charge on them, the charged droplets were attracted to the positive plate. Changing the strength of the electrical field offset the attraction and allowed Millikan to determine the charge of the electrons. • He measured the charge of the electrons (1. 60 X 10 -19 coulombs) and later used this to calculate their extremely small mass (9. 11 x 10 -28 grams).

2. 2

What did it look like inside?

Discover of the Nucleus • Ernest Rutherford

J. J. Thompson

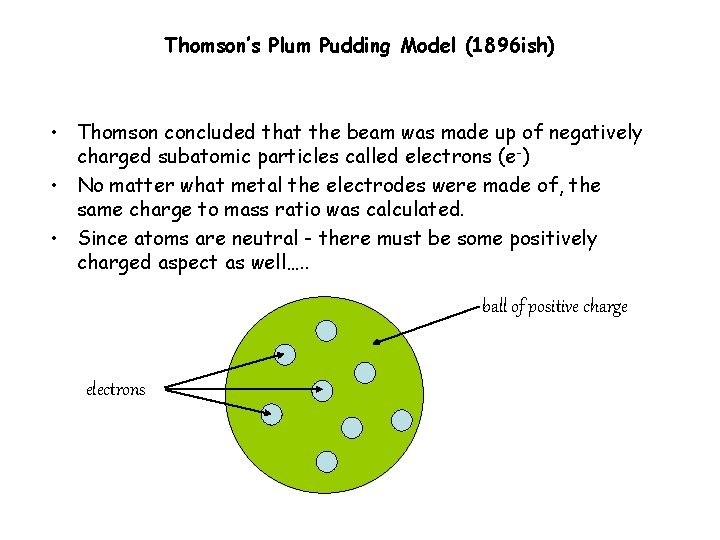
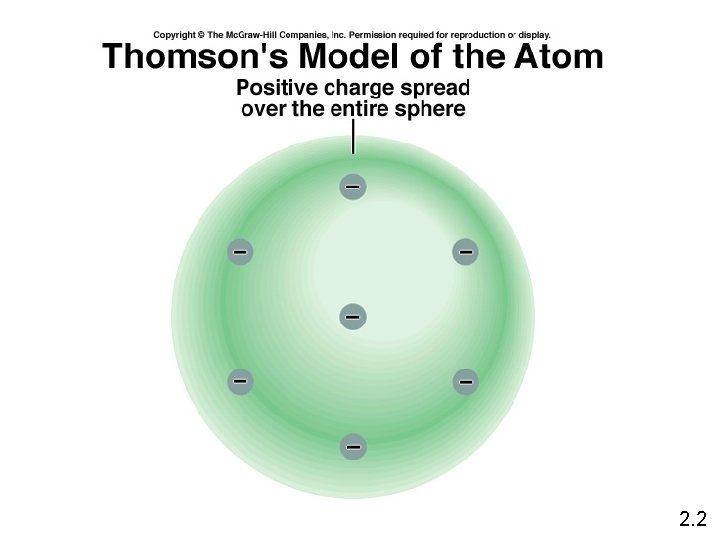
Thomson’s Plum Pudding Model (1896 ish) • Thomson concluded that the beam was made up of negatively charged subatomic particles called electrons (e -) • No matter what metal the electrodes were made of, the same charge to mass ratio was calculated. • Since atoms are neutral - there must be some positively charged aspect as well…. . ball of positive charge electrons

2. 2

Ernest Rutherford

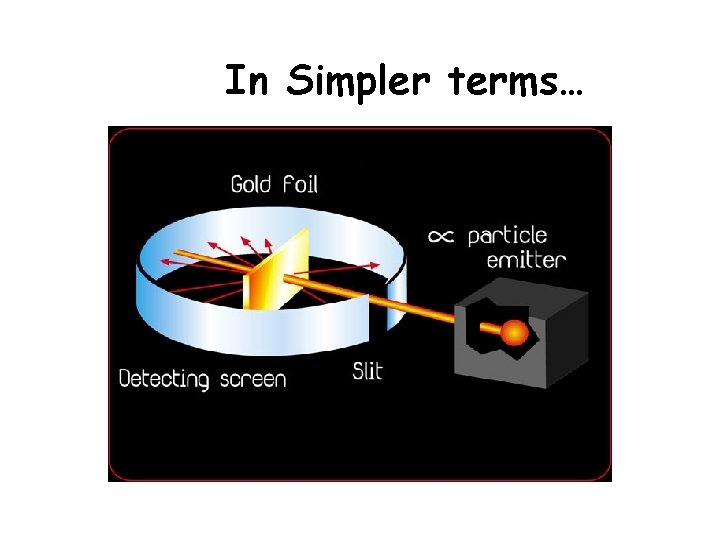
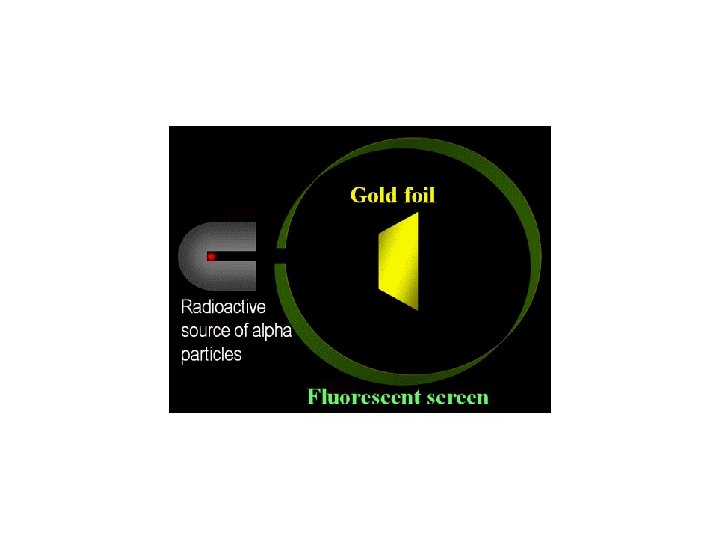
In Simpler terms…


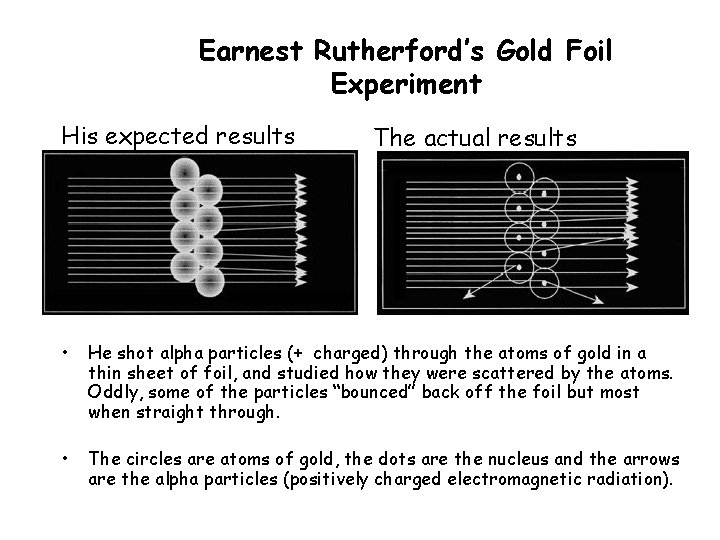
Earnest Rutherford’s Gold Foil Experiment His expected results The actual results • He shot alpha particles (+ charged) through the atoms of gold in a thin sheet of foil, and studied how they were scattered by the atoms. Oddly, some of the particles “bounced” back off the foil but most when straight through. • The circles are atoms of gold, the dots are the nucleus and the arrows are the alpha particles (positively charged electromagnetic radiation).

Rutherford’s Gold Foil Experiment ITS Chemistry

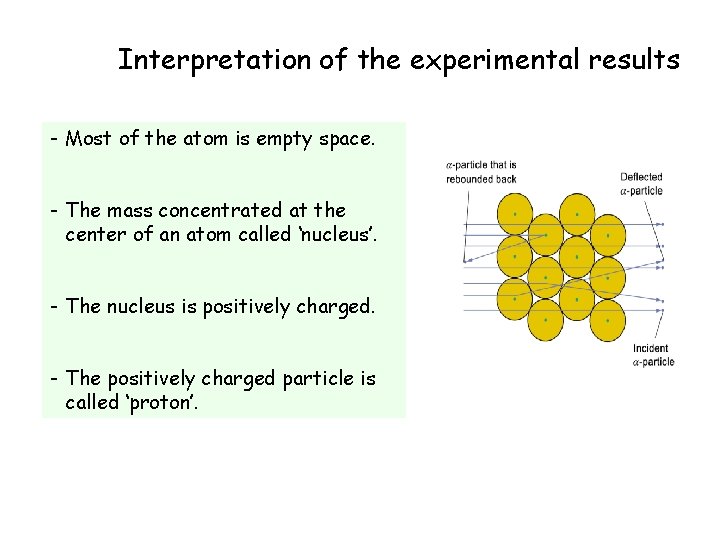
Interpretation of the experimental results - Most of the atom is empty space. - The mass concentrated at the center of an atom called ‘nucleus’. - The nucleus is positively charged. - The positively charged particle is called ‘proton’.

Discovery of the neutron • James Chadwick

James Chadwick

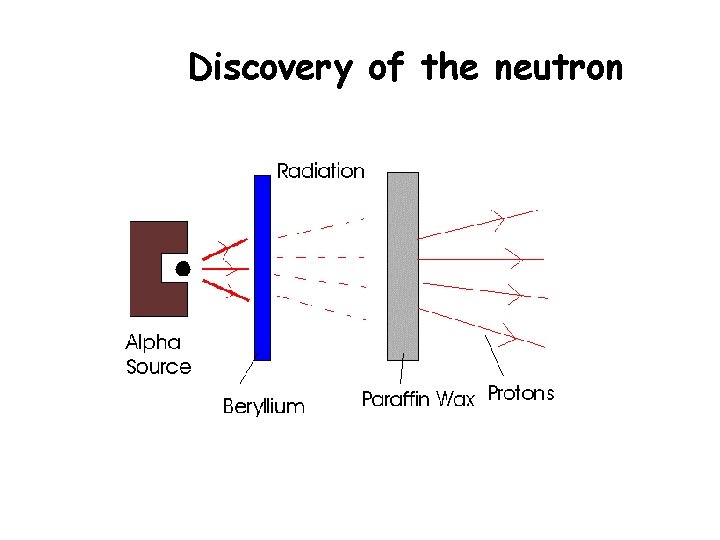
Discovery of the neutron

The Atom as we know it today

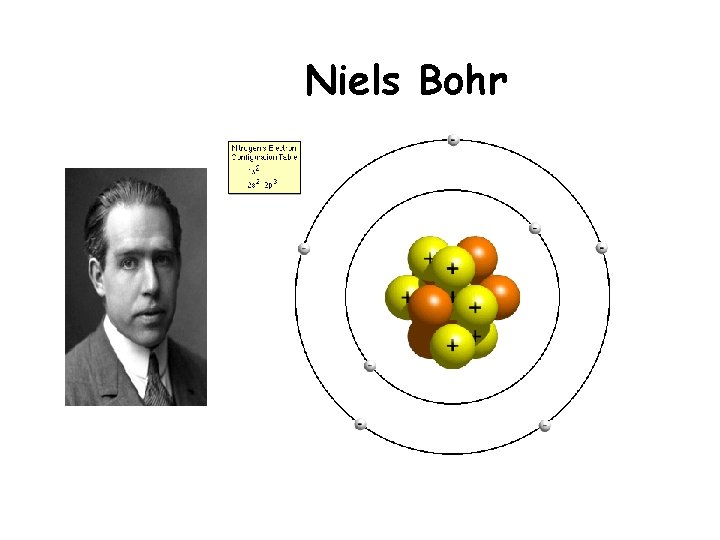
Niels Bohr