The History of Atomic Theory The discovery of














- Slides: 24

The History of Atomic Theory

• The discovery of the atom as we know it today was a progression. • Many scientists contributed to the development of modern day atomic theory.

Democritus (~460 BC ~370 BC) • Greece • Believed all matter was composed indivisible particles • “atomos” • Ideas were rejected by society (but correct)

Aristotle (~322 BC) • Greece • Believed everything in universe was composed of four elements: earth, fire, water, air • Ideas accepted by society (but incorrect)

John Dalton (1766 -1844) • England • Chemist

Dalton’s Atomic Theory 1. All matter is composed of atoms. Atoms are indivisible (didn’t know about protons, neutron, and electrons). 2. All atoms of an element are identical (didn’t know about isotopes). 3. Compounds are formed by two or more types of atoms (still true). 4. A chemical reaction is rearrangement of atoms (still true).

Dalton’s Billiard Ball Model of Atom • Solid sphere

J. J. Thomson (1856 -1940) • England • Physicist

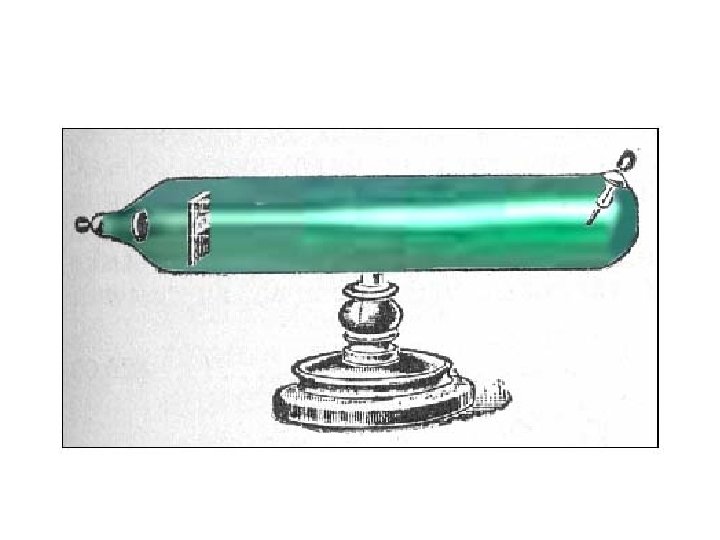
• In 1897, Thomson discovered the electron while studying cathode rays using cathode ray tubes


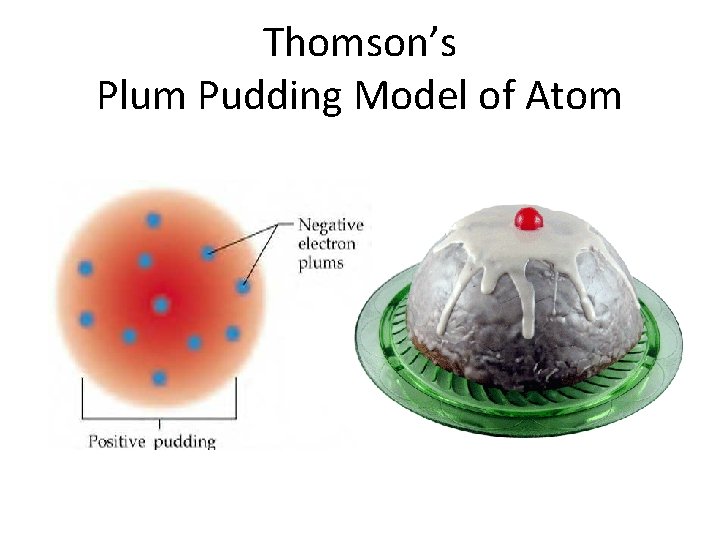
Thomson’s Plum Pudding Model of Atom

Robert Milliken (1868 -1953) • USA • Physicist

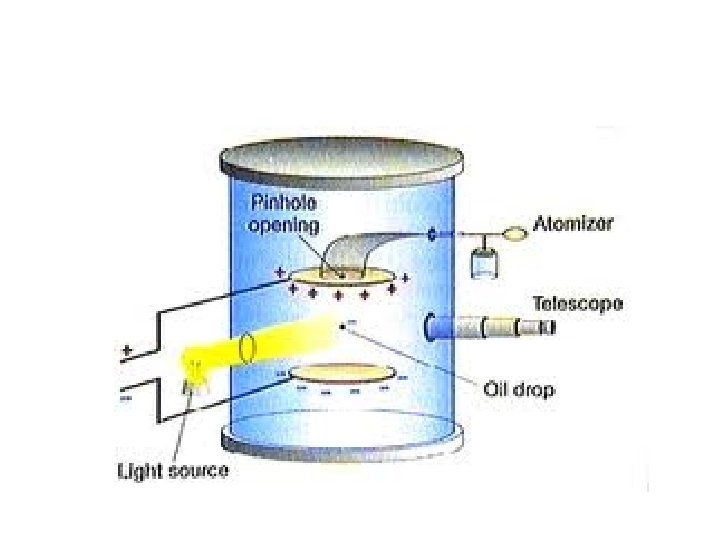
Milliken’s Oil Drop Experiment • 1909 • Discovered the charge on an electron


Ernest Rutherford (1871 -1937) • New Zealand • Physicist

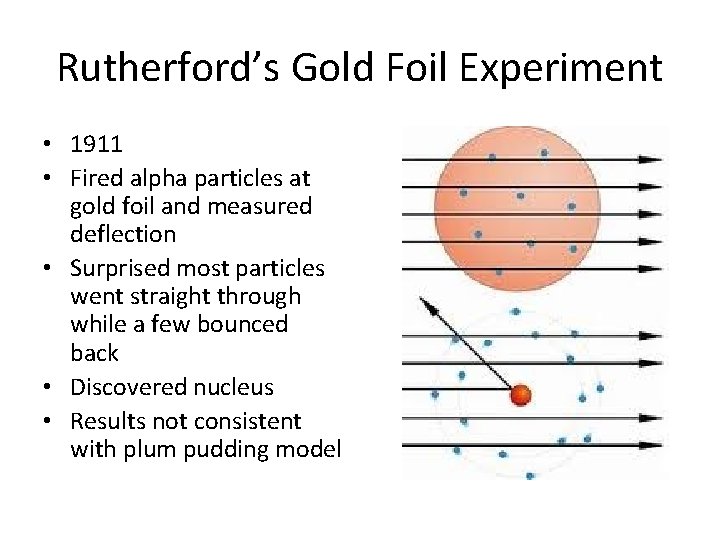
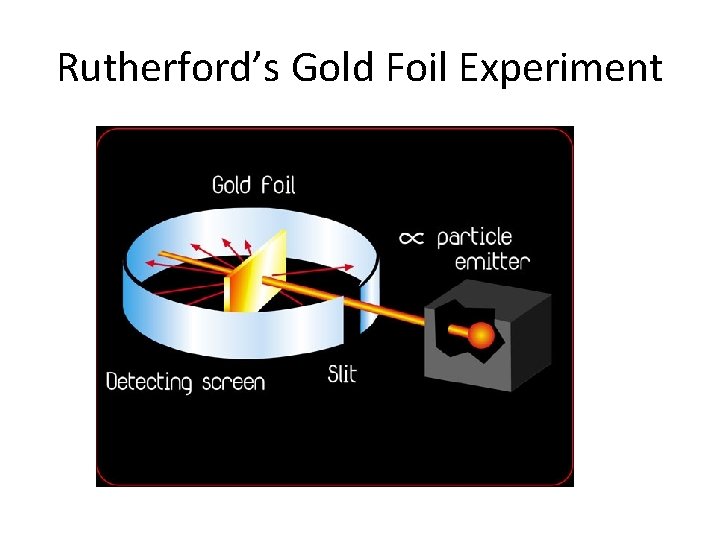
Rutherford’s Gold Foil Experiment • 1911 • Fired alpha particles at gold foil and measured deflection • Surprised most particles went straight through while a few bounced back • Discovered nucleus • Results not consistent with plum pudding model

Rutherford’s Gold Foil Experiment

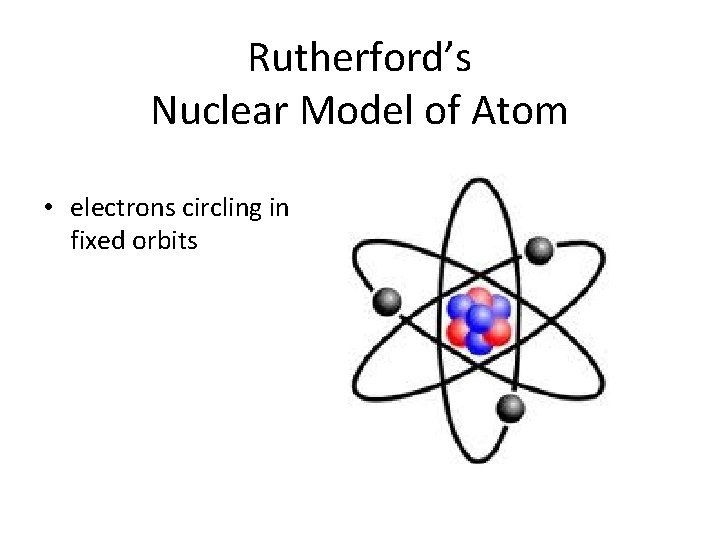
Rutherford’s Nuclear Model of Atom • electrons circling in fixed orbits

Niels Bohr (1885 -1962) • Denmark • Physicist

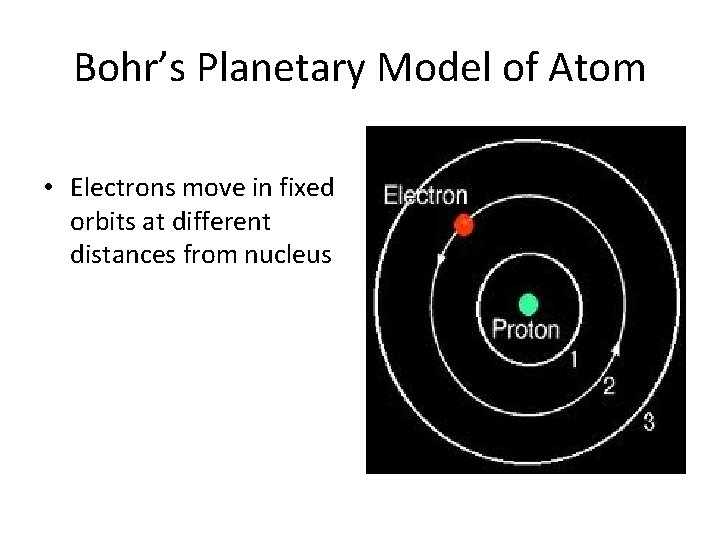
Bohr’s Planetary Model of Atom • Electrons move in fixed orbits at different distances from nucleus

Erwin Schrodinger (1887 -1961) • Austria • Physicist

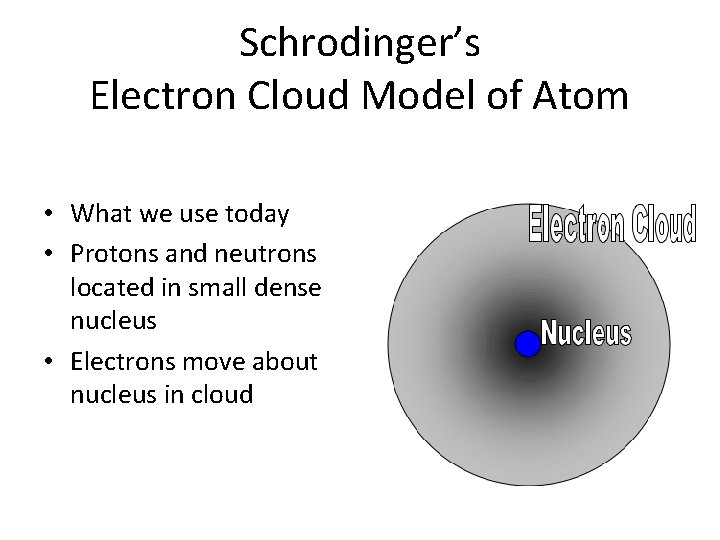
Schrodinger’s Electron Cloud Model of Atom • What we use today • Protons and neutrons located in small dense nucleus • Electrons move about nucleus in cloud

James Chadwick (1891 -1974) • England • Physicist

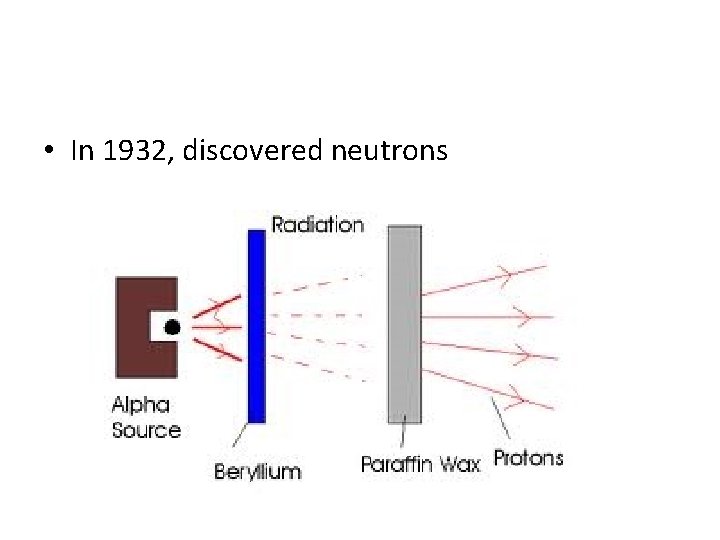
• In 1932, discovered neutrons