The Heart Outcomes Prevention Evaluation HOPE 3 Trial






- Slides: 21

The Heart Outcomes Prevention Evaluation (HOPE) – 3 Trial Eva Lonn, Jackie Bosch, Salim Yusuf For the HOPE-3 Investigators Population Health Research Institute, (PHRI) Mc. Master University and Hamilton Health Sciences, Hamilton, Canada Unrestricted grants from the Canadian Institutes of Health Research and Astra. Zeneca

Unique Aspects of HOPE-3 • BP lowering trial with wide range of BP entry criteria • Cholesterol lowering treatment based on risk opposed to baseline LDL or HDL measurement • Diverse population 4

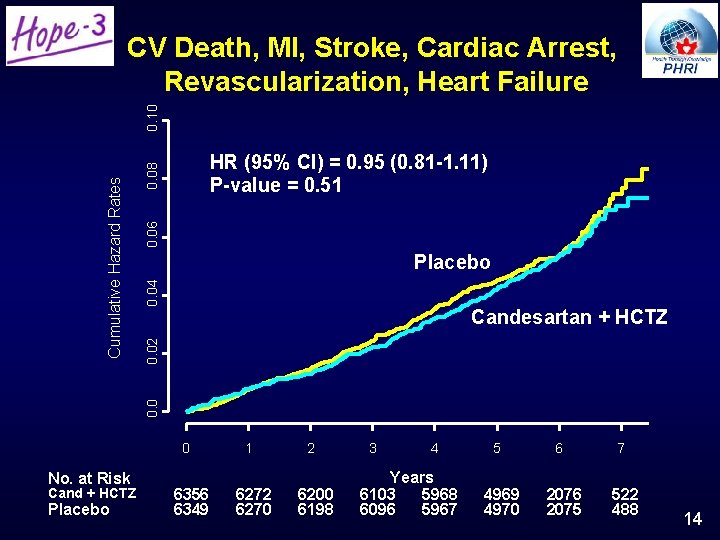
0. 06 0. 08 HR (95% CI) = 0. 95 (0. 81 -1. 11) P-value = 0. 51 0. 04 Placebo 0. 02 Candesartan + HCTZ 0. 0 Cumulative Hazard Rates 0. 10 CV Death, MI, Stroke, Cardiac Arrest, Revascularization, Heart Failure No. at Risk Cand + HCTZ Placebo 0 1 2 6356 6349 6272 6270 6200 6198 3 4 Years 6103 5968 6096 5967 5 4969 4970 6 2076 2075 7 522 488 14

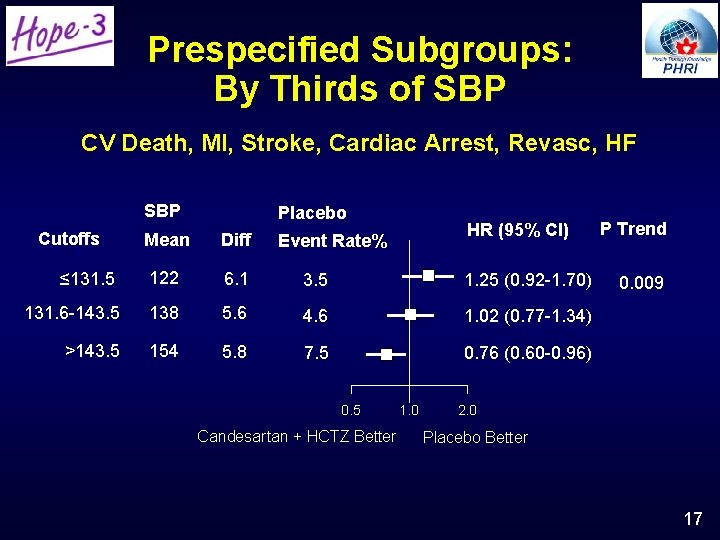
Prespecified Subgroups: By Thirds of SBP CV Death, MI, Stroke, Cardiac Arrest, Revasc, HF SBP Cutoffs Mean Placebo Diff HR (95% CI) Event Rate% ≤ 131. 5 122 6. 1 3. 5 1. 25 (0. 92 -1. 70) 131. 6 -143. 5 138 5. 6 4. 6 1. 02 (0. 77 -1. 34) >143. 5 154 5. 8 7. 5 0. 76 (0. 60 -0. 96) 0. 5 Candesartan + HCTZ Better 1. 0 P Trend 0. 009 2. 0 Placebo Better 17

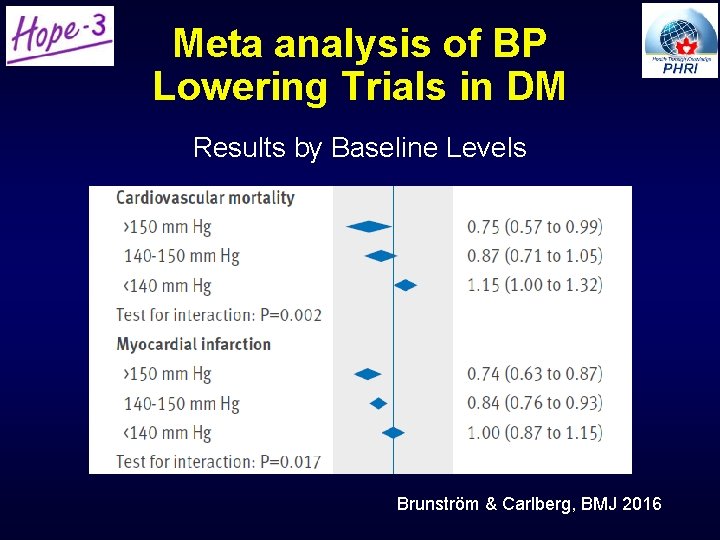
Meta analysis of BP Lowering Trials in DM Results by Baseline Levels Brunström & Carlberg, BMJ 2016

BP Lowering Arm: Conclusions • Fixed dose combination of Candesartan 16 mg + HCTZ 12. 5 mg/day reduced BP by 6. 0/3. 0 mm. Hg, but did not reduce CV events • CV events were significantly reduced in the highest third of SBP – SBP >143. 5 mm. Hg, mean 154 mm. Hg • Results were neutral in the middle third, and trended towards harm in the lowest third of SBP • Treatment increased lightheadedness, but not syncope or renal dysfunction 19

Cholesterol Lowering Arm Results Jackie Bosch 20

Unique Aspects of Cholesterol Lowering Arm • No entry criteria based on lipid level • No routine monitoring • No dose titration • Low dose of rosuvastatin

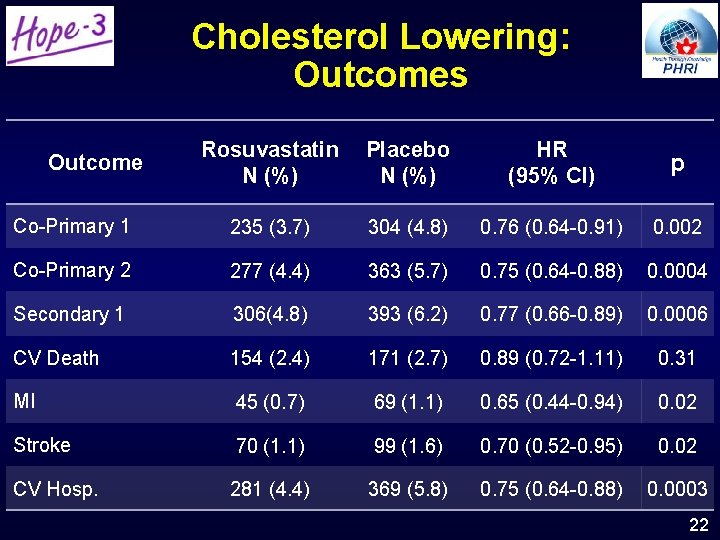
Cholesterol Lowering: Outcomes Rosuvastatin N (%) Placebo N (%) HR (95% CI) p Co-Primary 1 235 (3. 7) 304 (4. 8) 0. 76 (0. 64 -0. 91) 0. 002 Co-Primary 2 277 (4. 4) 363 (5. 7) 0. 75 (0. 64 -0. 88) 0. 0004 Secondary 1 306(4. 8) 393 (6. 2) 0. 77 (0. 66 -0. 89) 0. 0006 CV Death 154 (2. 4) 171 (2. 7) 0. 89 (0. 72 -1. 11) 0. 31 MI 45 (0. 7) 69 (1. 1) 0. 65 (0. 44 -0. 94) 0. 02 Stroke 70 (1. 1) 99 (1. 6) 0. 70 (0. 52 -0. 95) 0. 02 CV Hosp. 281 (4. 4) 369 (5. 8) 0. 75 (0. 64 -0. 88) 0. 0003 Outcome 22

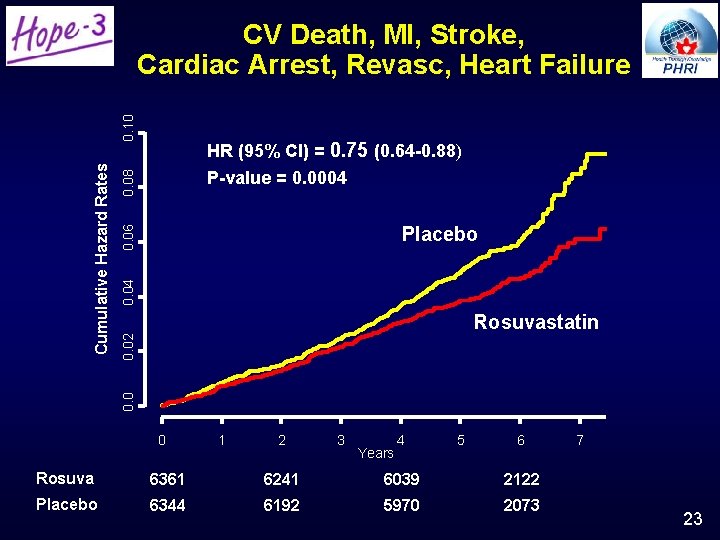
0. 08 HR (95% CI) = 0. 75 (0. 64 -0. 88) P-value = 0. 0004 0. 06 Placebo 0. 02 Rosuvastatin 0. 0 Cumulative Hazard Rates 0. 10 CV Death, MI, Stroke, Cardiac Arrest, Revasc, Heart Failure 0 1 2 3 Years 4 5 6 Rosuva 6361 6241 6039 2122 Placebo 6344 6192 5970 2073 7 23

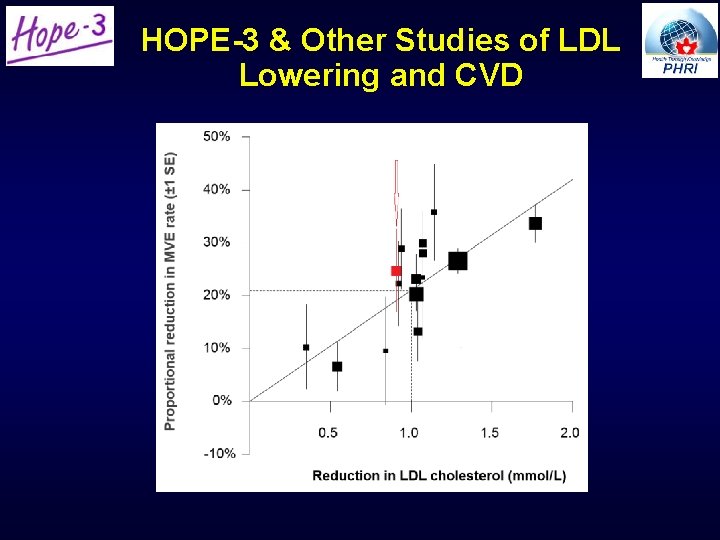
HOPE-3 & Other Studies of LDL Lowering and CVD

Cholesterol Lowering: Conclusions • Rosuvastatin 10 mg/day reduced: – LDL-C by 34. 6 mg/dl (0. 9 mmol/l; i. e. 27% in LDL-C) – CVD by 25% • Consistent benefits regardless of: – LDL-C – SBP – Risk – CRP – Ethnicity • Excess in muscle pain/weakness (reversible) and perhaps cataract surgery • No excess in rhabdomyolysis, myopathy or new diabetes 27

Combined BP & Cholesterol Lowering vs Double Placebo Salim Yusuf 28

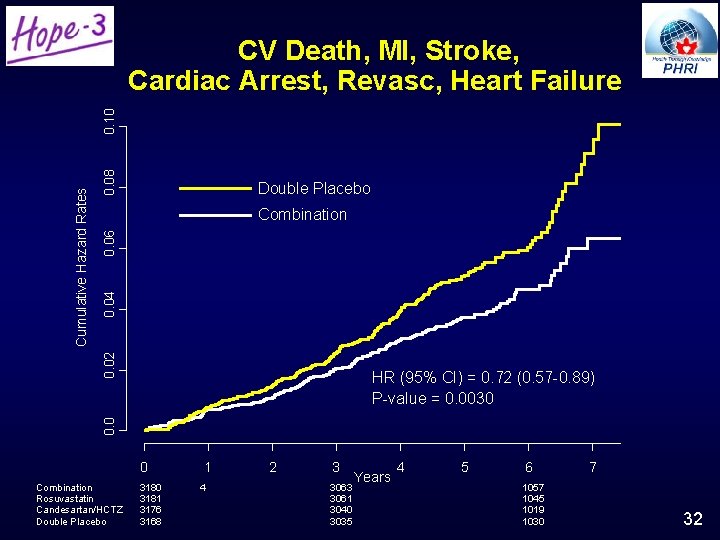
Unique Aspects of BP & Chol Lowering • First formal testing of polypill concept on clinical events • Demonstrates that the concept is valid in people with elevated BP; in others there is no benefit

0. 08 Double Placebo 0. 04 0. 06 Combination 0. 02 Cumulative Hazard Rates 0. 10 CV Death, MI, Stroke, Cardiac Arrest, Revasc, Heart Failure 0. 0 HR (95% CI) = 0. 72 (0. 57 -0. 89) P-value = 0. 0030 0 Combination Rosuvastatin Candesartan/HCTZ Double Placebo 3180 3181 3176 3168 1 4 2 3 Years 3063 3061 3040 3035 4 5 6 1057 1045 1019 1030 7 32

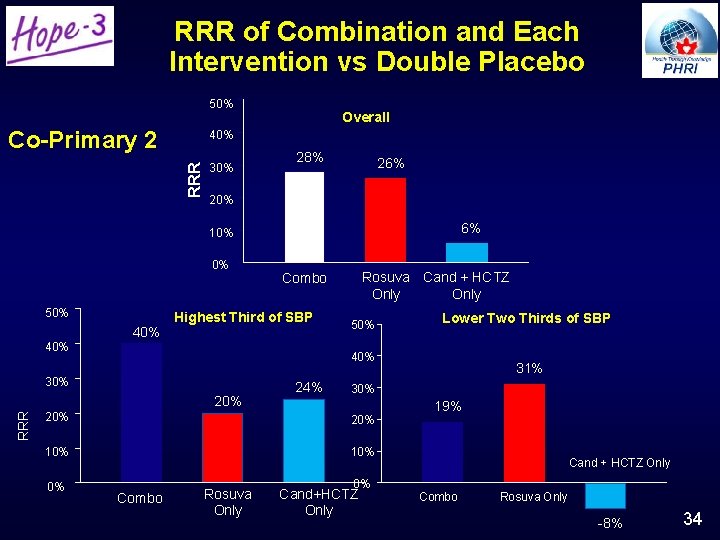
RRR of Combination and Each Intervention vs Double Placebo 50% Co-Primary 2 Overall RRR 40% 30% 28% 26% 20% 6% 10% 0% 50% 40% Highest Third of SBP 40% 50% 24% 20% 10% Combo Rosuva Only 31% 30% 20% 0% Lower Two Thirds of SBP 40% 30% RRR Rosuva Cand + HCTZ Only Combo 0% Cand+HCTZ Only 19% Cand + HCTZ Only Combo Rosuva Only -8% 34

Clinical Implications • Statins beneficial in intermediate-risk individuals without CVD • BP lowering benefits only those with elevated BP • Combined BP & cholesterol lowering: – Leads to a 40% risk reduction in hypertensives (benefits from both BP lowering and statin) • In others, 30% RRR from statin alone • Pragmatic strategy: – No Lipid or BP entry criteria or targets – No Dose titration – Infrequent safety monitoring Strategy used in HOPE-3 is simple, safe and effective and widely applicable 37

Back up

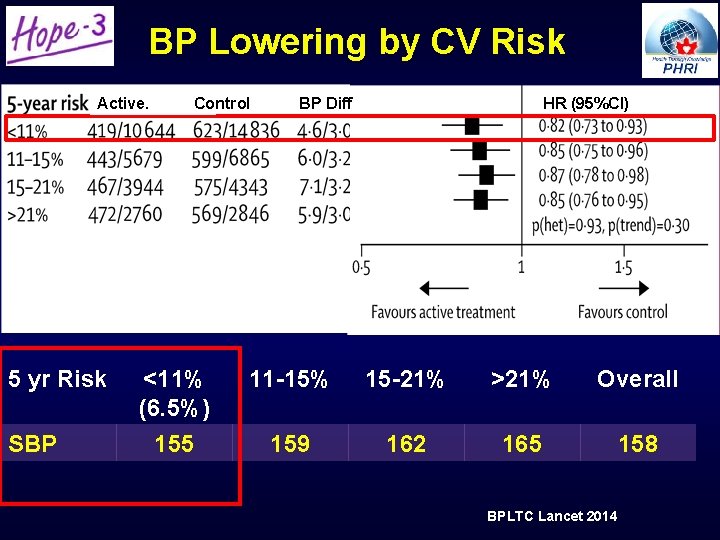
BP Lowering by CV Risk Active. 5 yr Risk SBP Control <11% (6. 5%) 155 BP Diff HR (95%CI) 11 -15% 15 -21% >21% Overall 159 162 165 158 BPLTC Lancet 2014

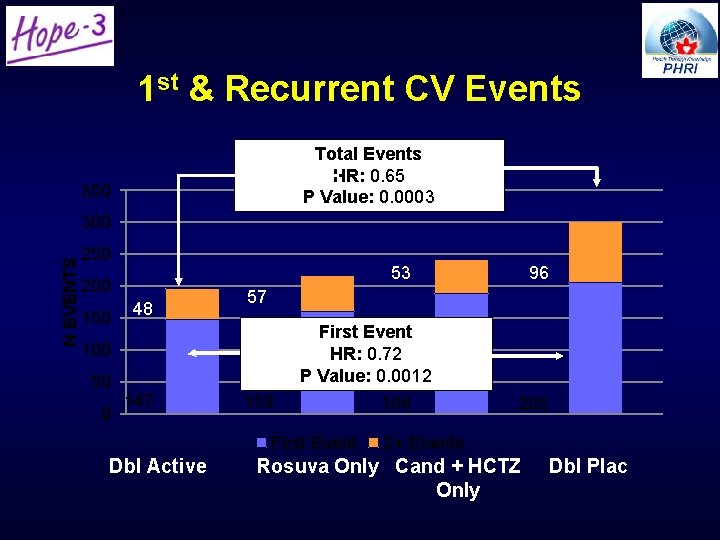
1 st & Recurrent CV Events Total Events HR: 0. 65 P Value: 0. 0003 350 N EVENTS 300 250 53 200 150 48 57 First Event HR: 0. 72 P Value: 0. 0012 100 50 0 96 147 159 First Event Dbl Active 188 205 2+ Events Rosuva Only Cand + HCTZ Only Dbl Plac

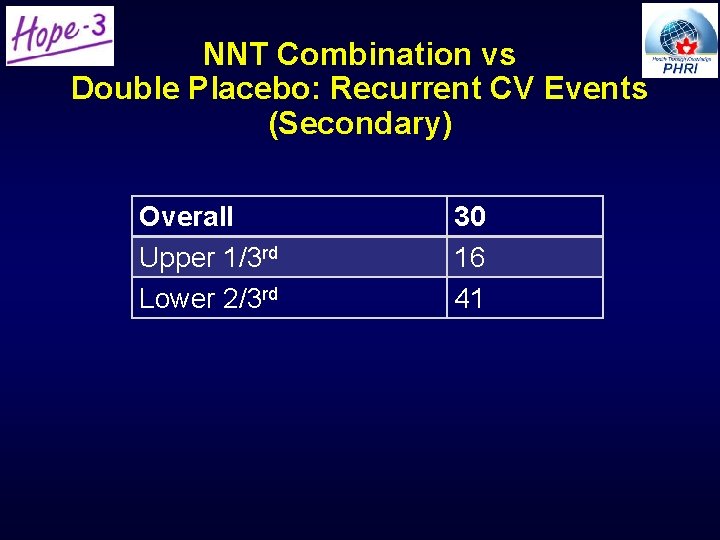
NNT Combination vs Double Placebo: Recurrent CV Events (Secondary) Overall Upper 1/3 rd Lower 2/3 rd 30 16 41