The halogens Later Group 7 The Halogens Physical




- Slides: 12

The halogens Later

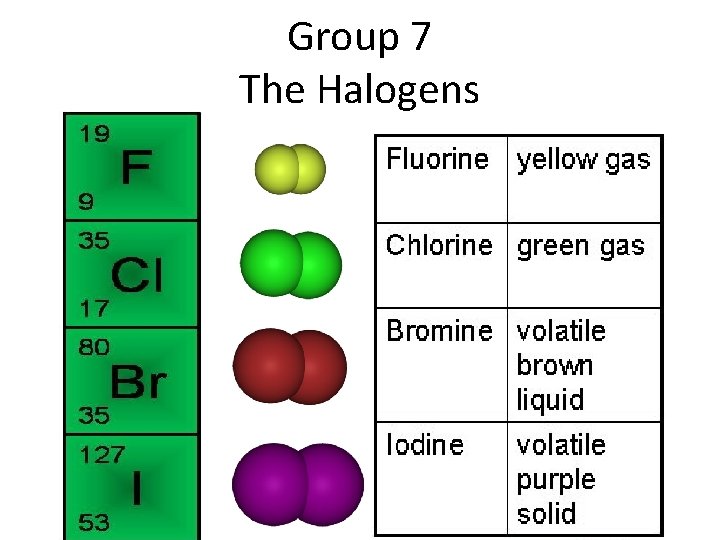
Group 7 The Halogens

Physical Properties Simple Molecular Structures • • • Diatomic molecules Strong covalent bonds Weak intermolecular forces Low melting and boiling points Poor conductors of heat and electricity Melting point and boiling point increase as you go down the group.

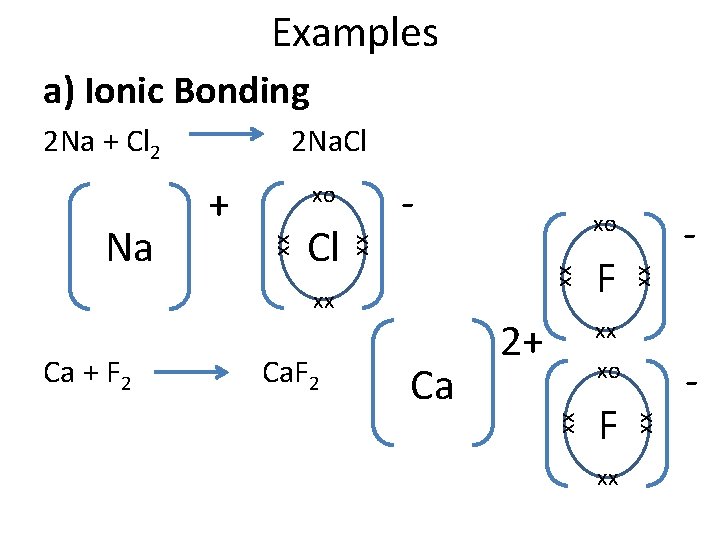
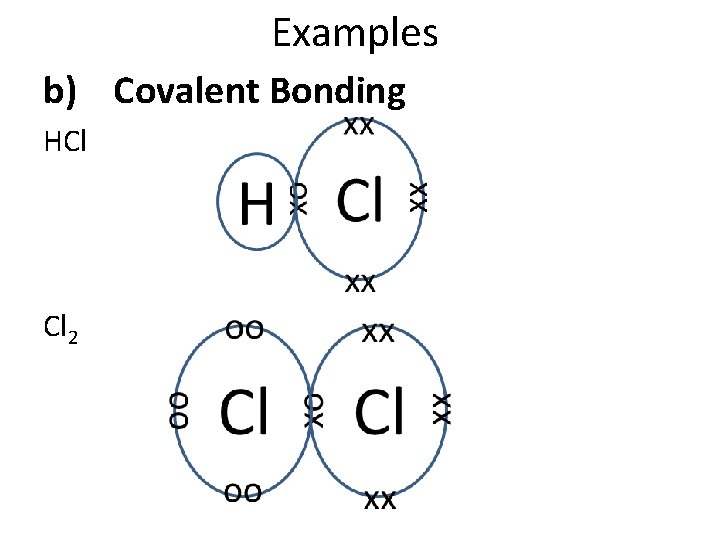
Chemical Properties of the Halogens All have 7 electrons in their outer shell. They all want to gain one more electron for a full outer shell. There are two ways they can do this: a) Ionic bonding – Accept one electron from a metal atom to become a halide ion with a -1 charge. b) Covalent bonding – Form one covalent bond with another non-metal.

Examples a) Ionic Bonding - Ca + F 2 Ca 2+ xx - xo F xx xx xx F xx xx Cl - xo xx + xo xx Na 2 Na. Cl xx 2 Na + Cl 2

Examples b) Covalent Bonding HCl Cl 2

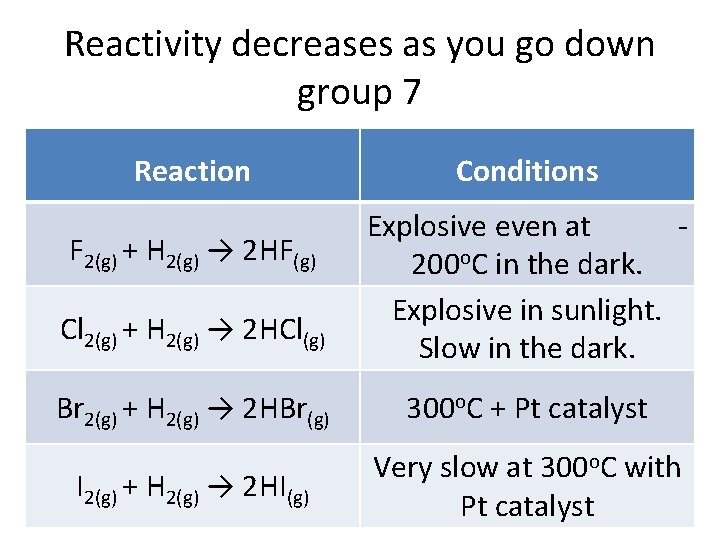
Reactivity decreases as you go down group 7 Reaction Conditions Cl 2(g) + H 2(g) → 2 HCl(g) Explosive even at 200 o. C in the dark. Explosive in sunlight. Slow in the dark. Br 2(g) + H 2(g) → 2 HBr(g) 300 o. C + Pt catalyst I 2(g) + H 2(g) → 2 HI(g) Very slow at 300 o. C with Pt catalyst F 2(g) + H 2(g) → 2 HF(g)

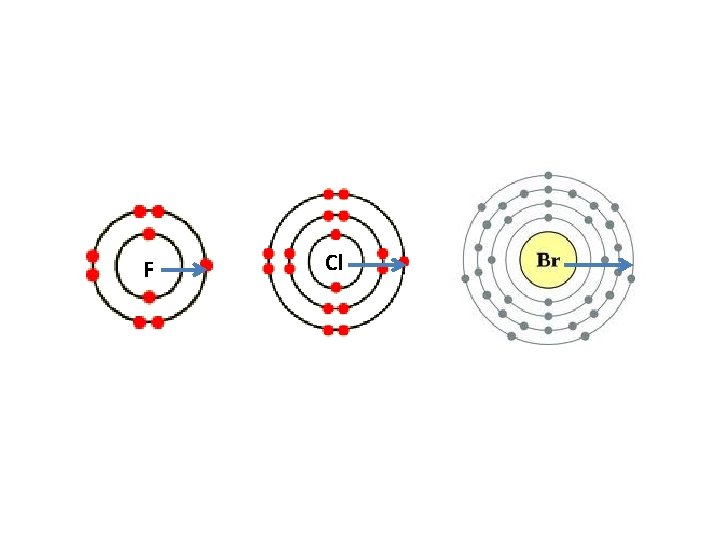
Group 7 – forming negative ions • Reactivity decreases as you go down the group. • In larger atoms the outermost electrons are further from the nucleus. • This makes it harder to gain an electron as it will be less strongly attracted to the nucleus R E A C T I V I T Y I N C R E A S E S F Cl

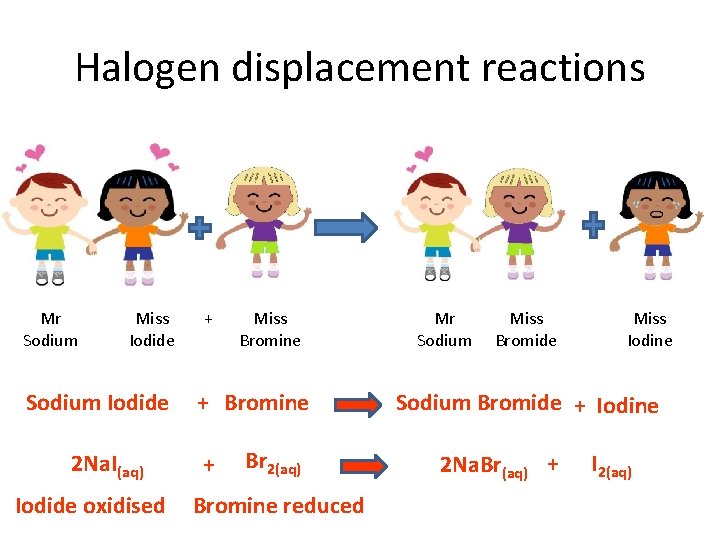
Halogen displacement reactions Mr Sodium Miss Iodide Sodium Iodide 2 Na. I(aq) Iodide oxidised + Miss Bromine + Br 2(aq) Bromine reduced Mr Sodium Miss Bromide Miss Iodine Sodium Bromide + Iodine 2 Na. Br(aq) + I 2(aq)

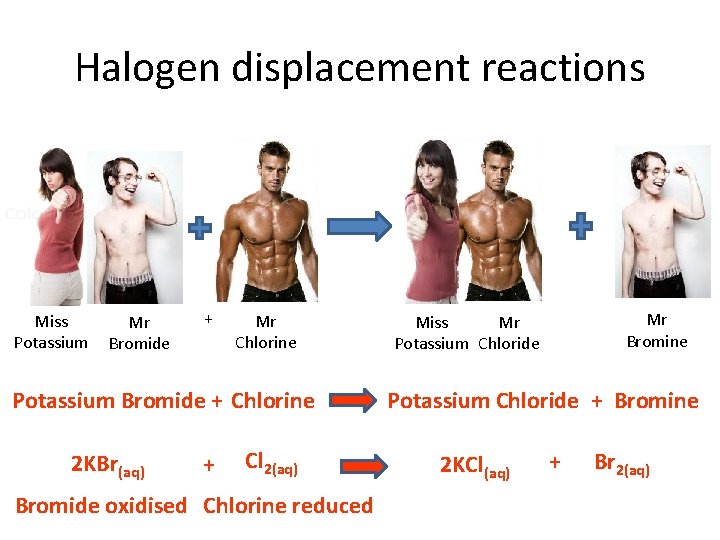
Halogen displacement reactions Miss Potassium Mr Bromide + Mr Chlorine Potassium Bromide + Chlorine 2 KBr(aq) + Cl 2(aq) Bromide oxidised Chlorine reduced Mr Bromine Miss Mr Potassium Chloride + Bromine 2 KCl(aq) + Br 2(aq)

What if we add fluorine to aqueous potassium chloride? Fluorine is more reactive than chlorine so would displace chloride from it’s salt. However, fluorine is so reactive it would instantly react with the water before it got to the chloride ions!

F Cl