The Greenhouse Effect Terrestrial Emissions Terrestrial emissions have








- Slides: 15

The Greenhouse Effect

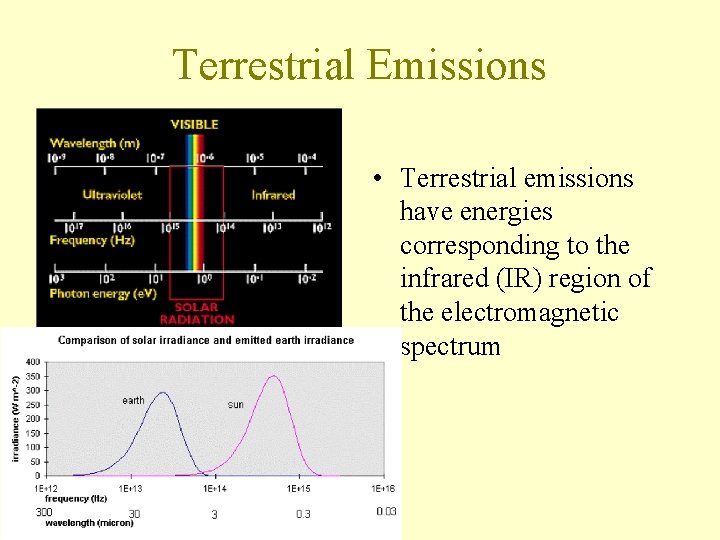
Terrestrial Emissions • Terrestrial emissions have energies corresponding to the infrared (IR) region of the electromagnetic spectrum

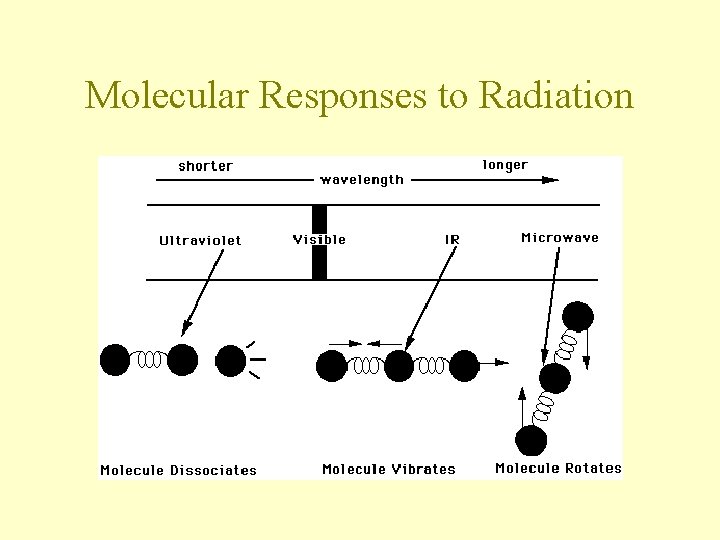
Visible & UV Radiation • Certain energies cause electrons to be excited into higher energy orbitals • Very energetic photons (uv to x-ray) may cause electrons to be ejected from molecule (ionization)

IR Radiation • Lower in energy than visible radiation • Does not possess enough energy to eject electrons • Absorbed IR radiation can excite vibrations in molecules


Molecular Responses to Radiation

What Are Molecular Vibrations? • Consider a diatomic molecule as being 2 balls connected by a spring • When the molecule vibrates, the balls move towards and away from each other at a certain frequency • The energy of the system is related to the amount the spring stretches or compresses

• The frequency of the vibration is proportional to the square root of the ratio of the spring force constant to the masses on the string. – the lighter the masses on the spring, the higher the vibrational frequency – the tighter the spring, the higher the vibrational frequency • Vibrational frequencies for stretching bonds in molecules are related to: – bond strength – masses of atoms

• Molecules differ from balls & springs because their vibrational frequencies are quantized • Only certain energies are allowed • only photons of certain energies will excite molecular vibrations

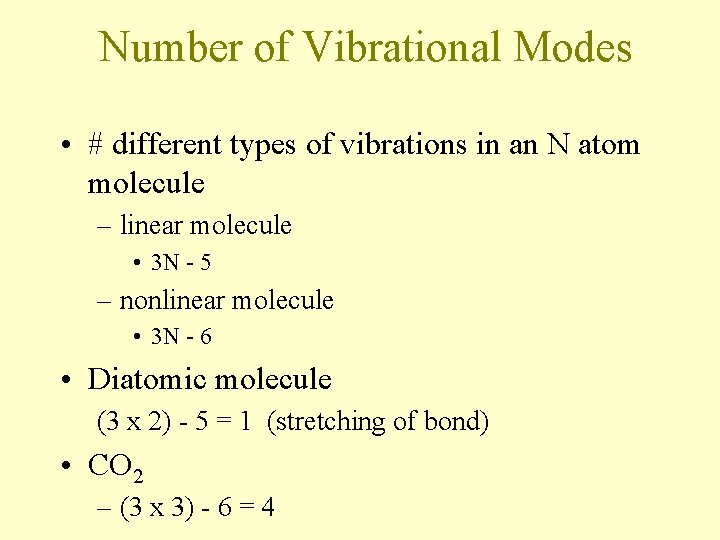
Number of Vibrational Modes • # different types of vibrations in an N atom molecule – linear molecule • 3 N - 5 – nonlinear molecule • 3 N - 6 • Diatomic molecule (3 x 2) - 5 = 1 (stretching of bond) • CO 2 – (3 x 3) - 6 = 4

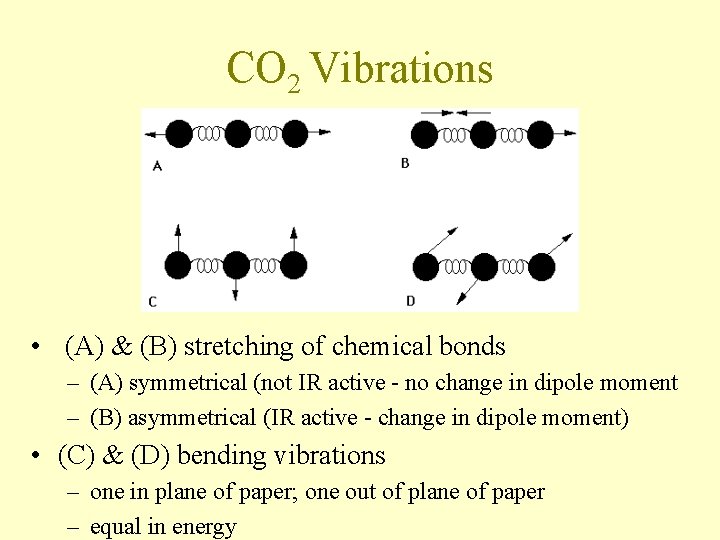
CO 2 Vibrations • (A) & (B) stretching of chemical bonds – (A) symmetrical (not IR active - no change in dipole moment – (B) asymmetrical (IR active - change in dipole moment) • (C) & (D) bending vibrations – one in plane of paper; one out of plane of paper – equal in energy

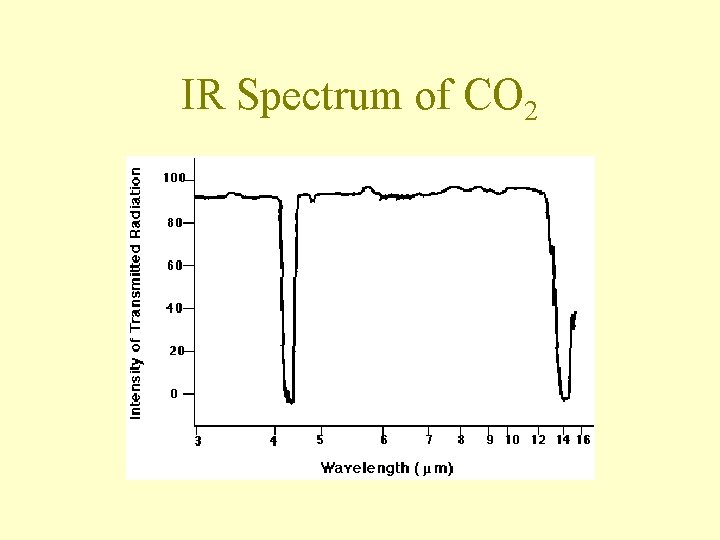
IR Spectrum of CO 2

General Trends • The stronger the bond, the more energy required to excite the stretching vibration – frequency decreases in the order triple bond > double bond > single bond • The heavier an atom, the lower the frequencies of vibrations involving that atom

General Trends • Polar molecules and molecules with polar bonds capture IR photons more efficiently than nonpolar molecules • All molecules with three or more atoms absorb IR radiation because they all have some vibrations that change the polarity of the molecul

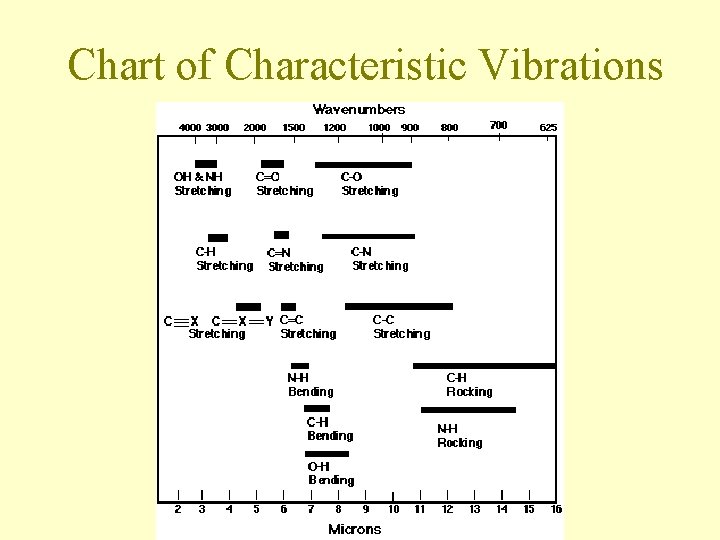
Chart of Characteristic Vibrations
 Greenhouse effect long and shortwave radiation
Greenhouse effect long and shortwave radiation Why is the greenhouse effect important
Why is the greenhouse effect important Why is the greenhouse effect important
Why is the greenhouse effect important Greenhouse effect
Greenhouse effect Greenhouse effect
Greenhouse effect Enhanced greenhouse effect
Enhanced greenhouse effect Slidetodoc
Slidetodoc Global warming 101
Global warming 101 Natural greenhouse effect
Natural greenhouse effect Greenhouse effect
Greenhouse effect Greenhouse effect on plants
Greenhouse effect on plants Greenhouse effect
Greenhouse effect Greenhouse effect powerpoint
Greenhouse effect powerpoint Causes of greenhouse effect
Causes of greenhouse effect Greenhouse effect in order
Greenhouse effect in order Green home love nature
Green home love nature