The good the bad and the ugly Use

The good, the bad , and the ugly : Use and abuse of nuclear physics • Nuclear power • Nuclear bombs • Nuclear waste Ch. 15 Wed. Apr. 11, 2006 Phy 107 Lecture 30 1

What does it take to generate 1 GW of power ? Hydroelectric power: 60, 000 tons of water per second. Burning Coal: 10, 000 tons of coal per day. Nuclear reactor: 100 tons of uranium per year. Wed. Apr. 11, 2006 Phy 107 Lecture 30 2
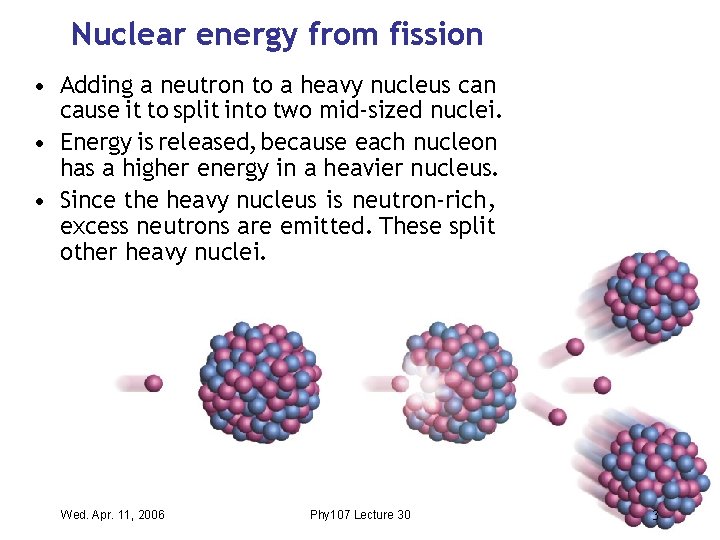
Nuclear energy from fission • Adding a neutron to a heavy nucleus can cause it to split into two mid-sized nuclei. • Energy is released, because each nucleon has a higher energy in a heavier nucleus. • Since the heavy nucleus is neutron-rich , excess neutrons are emitted. These split other heavy nuclei. Wed. Apr. 11, 2006 Phy 107 Lecture 30 3

Nuclear fuel • Fission (= splitting) of a heavy nucleus works with uranium (235 U) and plutonium (239 Pu). • Fission is initiated by neutrons , and it also produces more neutrons. • If this process remains under control, it can be used to generate nuclear power. • If not , a runaway ‘chain reaction’ occurs and splits more and more nuclei , eventually causing the meltdown of a nuclear reactor. Wed. Apr. 11, 2006 Phy 107 Lecture 30 4
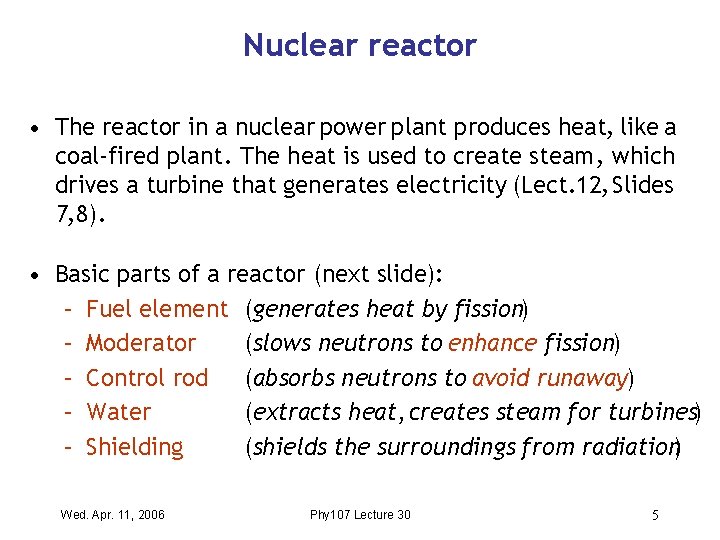
Nuclear reactor • The reactor in a nuclear power plant produces heat, like a coal-fired plant. The heat is used to create steam , which drives a turbine that generates electricity (Lect. 12, Slides 7, 8). • Basic parts of a reactor (next slide): – Fuel element (generates heat by fission) – Moderator (slows neutrons to enhance fission) – Control rod (absorbs neutrons to avoid runaway) – Water (extracts heat, creates steam for turbines) – Shielding (shields the surroundings from radiation) Wed. Apr. 11, 2006 Phy 107 Lecture 30 5
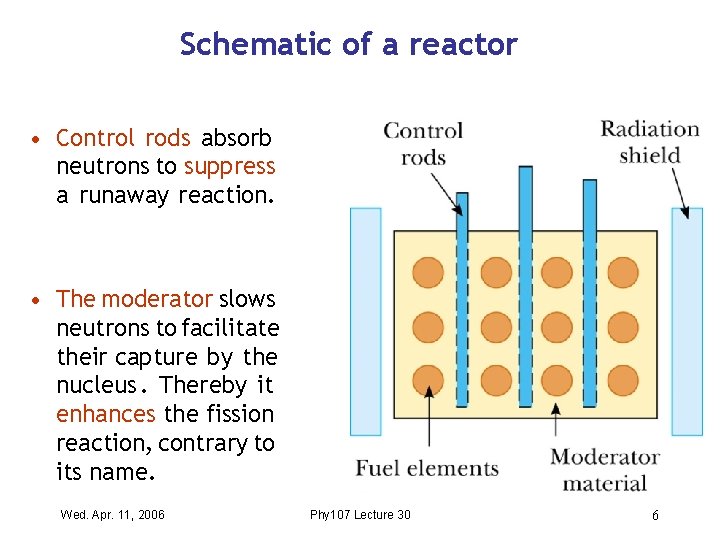
Schematic of a reactor • Control rods absorb neutrons to suppress a runaway reaction. • The moderator slows neutrons to facilitate their capture by the nucleus. Thereby it enhances the fission reaction, contrary to its name. Wed. Apr. 11, 2006 Phy 107 Lecture 30 6
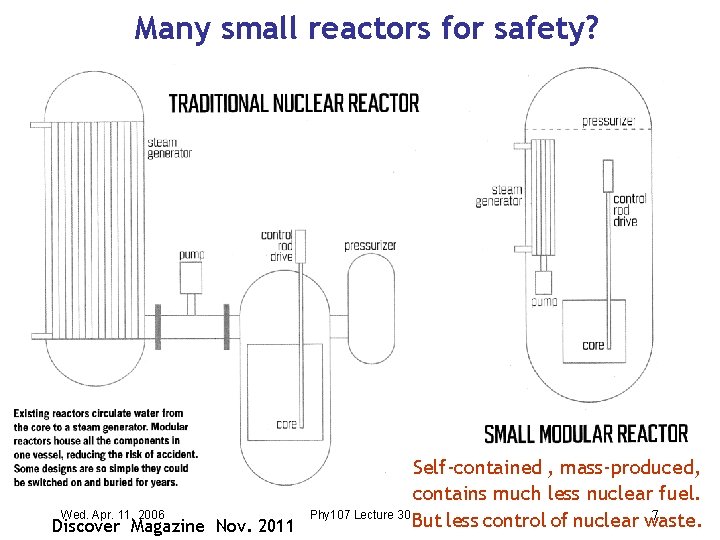
Many small reactors for safety? Self-contained , mass-produced, contains much less nuclear fuel. Wed. Apr. 11, 2006 Phy 107 Lecture 30 7 But less control of nuclear waste. Discover Magazine Nov. 2011
Breeder reactors • A breeder reactor produces more nuclear fuel than it uses. • Inert 238 U nuclei (see next slide) absorb neutrons and are transformed into plutonium (239 P), which is a nuclear fuel. • This design is able to utilize 100 % of the natural uranium. In standard reactors only 1 % is used (top of Slide 27) , and 99% becomes radioactive nuclear waste. • There is the risk that plutonium gets into the wrong hands. Therefore the US and many other countries don’t use such reactors commercially. Wed. Apr. 11, 2006 Phy 107 Lecture 30 8
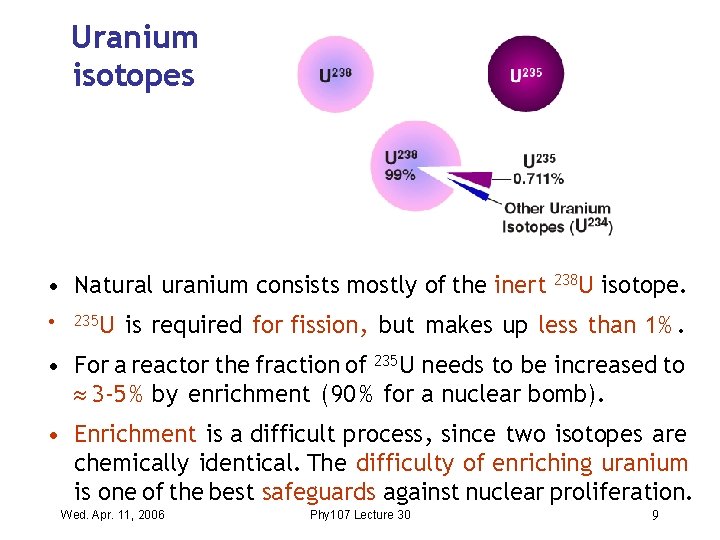
Uranium isotopes • Natural uranium consists mostly of the inert 238 U isotope. • 235 U is required for fission , but makes up less than 1%. • For a reactor the fraction of 235 U needs to be increased to 3 -5 % by enrichment ( 90 % for a nuclear bomb). • Enrichment is a difficult process , since two isotopes are chemically identical. The difficulty of enriching uranium is one of the best safeguards against nuclear proliferation. Wed. Apr. 11, 2006 Phy 107 Lecture 30 9
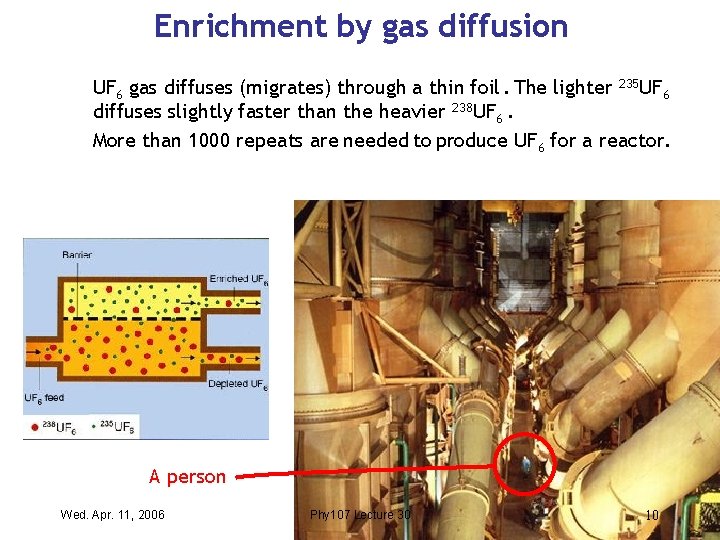
Enrichment by gas diffusion UF 6 gas diffuses (migrates) through a thin foil. The lighter 235 UF 6 diffuses slightly faster than the heavier 238 UF 6. More than 1000 repeats are needed to produce UF 6 for a reactor. A person Wed. Apr. 11, 2006 Phy 107 Lecture 30 10

The gas diffusion plant at Oak Ridge 12 000 workers produced 50 kg of highly-enriched Wed. Apr. 11, 2006 Phy 107 Lecture 30 235 U. 11
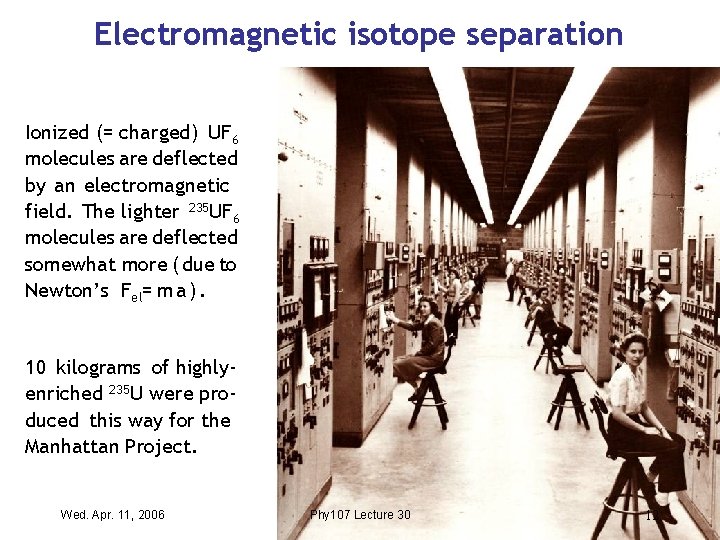
Electromagnetic isotope separation Ionized (= charged) UF 6 molecules are deflected by an electromagnetic field. The lighter 235 UF 6 molecules are deflected somewhat more ( due to Newton’s Fel= m a ). 10 kilograms of highlyenriched 235 U were produced this way for the Manhattan Project. Wed. Apr. 11, 2006 Phy 107 Lecture 30 12

Gas centrifuge enrichment Gaseous UF 6 is placed in a centrifuge, where rapid rotation flings heavier 238 U to the outer edge, leaving enriched 235 UF closer to the center. Many 6 centrifuges need to be cascaded for multiple repeats. This is the method of choice for producing enriched uranium these days (for example by Iran). Wed. Apr. 11, 2006 Phy 107 Lecture 30 13
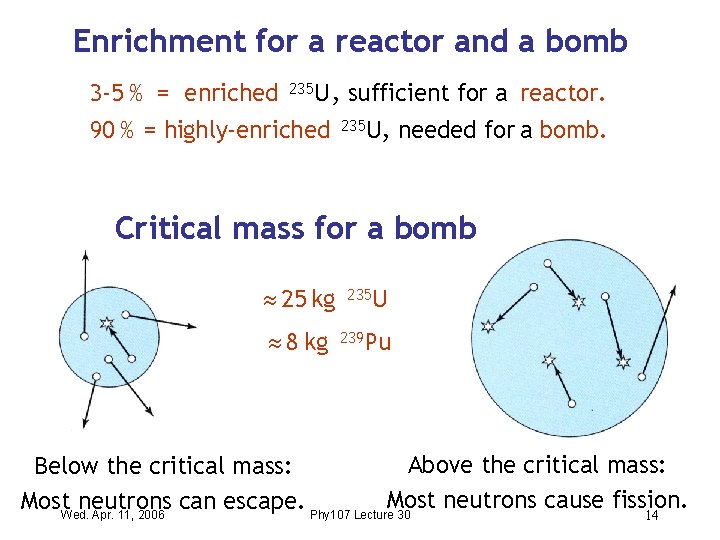
Enrichment for a reactor and a bomb 3 -5 % = enriched 235 U , 90 % = highly-enriched sufficient for a reactor. 235 U, needed for a bomb. Critical mass for a bomb 25 kg 235 U 8 kg 239 Pu Above the critical mass: Below the critical mass: neutrons cause fission. Most. Wed. neutrons can escape. Phy 107 Lecture. Most Apr. 11, 2006 30 14
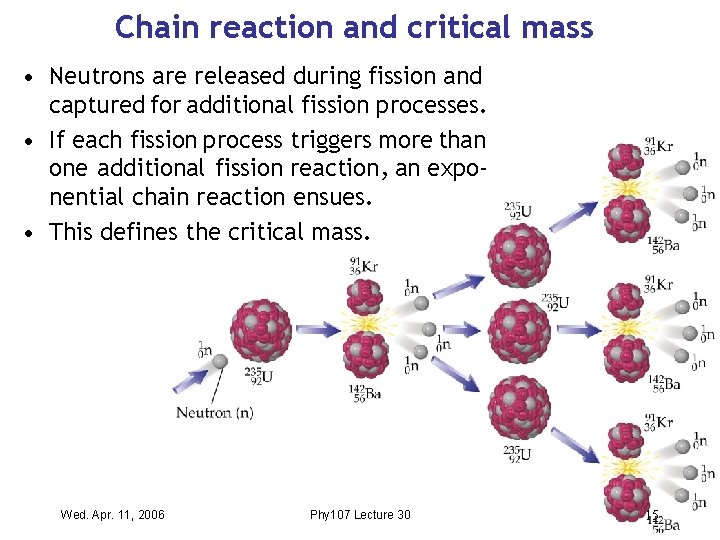
Chain reaction and critical mass • Neutrons are released during fission and captured for additional fission processes. • If each fission process triggers more than one additional fission reaction , an exponential chain reaction ensues. • This defines the critical mass. Wed. Apr. 11, 2006 Phy 107 Lecture 30 15
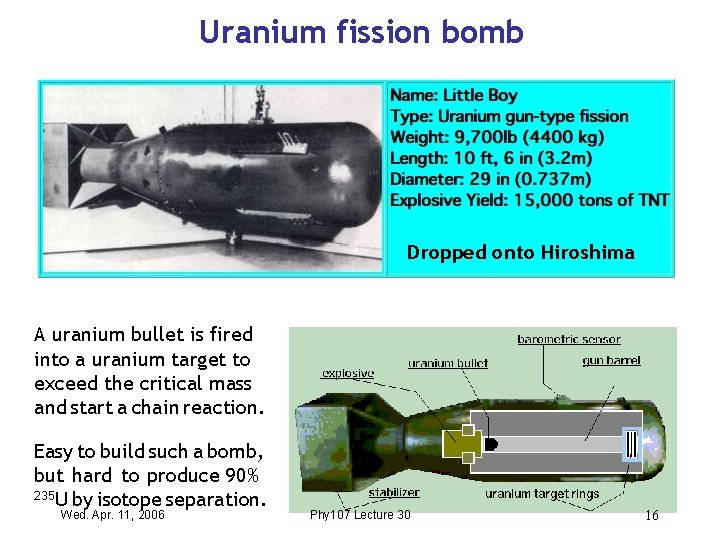
Uranium fission bomb Dropped onto Hiroshima A uranium bullet is fired into a uranium target to exceed the critical mass and start a chain reaction. Easy to build such a bomb, but hard to produce 90% 235 U by isotope separation. Wed. Apr. 11, 2006 Phy 107 Lecture 30 16
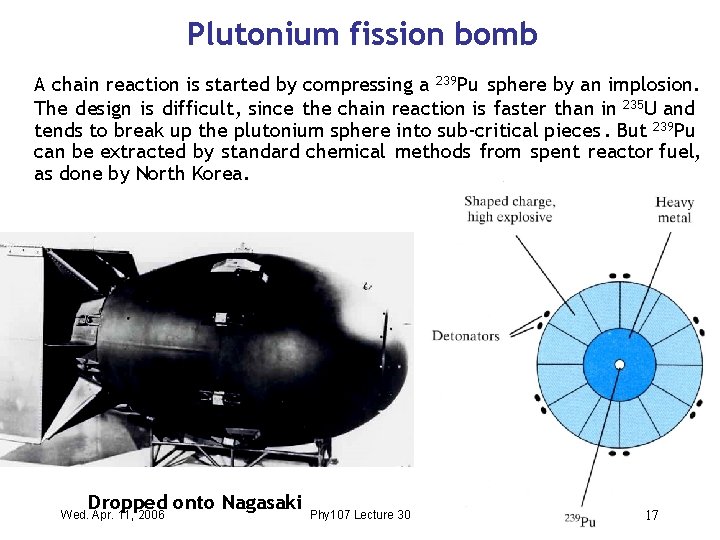
Plutonium fission bomb A chain reaction is started by compressing a 239 Pu sphere by an implosion. The design is difficult , since the chain reaction is faster than in 235 U and tends to break up the plutonium sphere into sub-critical pieces. But 239 Pu can be extracted by standard chemical methods from spent reactor fuel, as done by North Korea. Dropped onto Nagasaki Wed. Apr. 11, 2006 Phy 107 Lecture 30 17
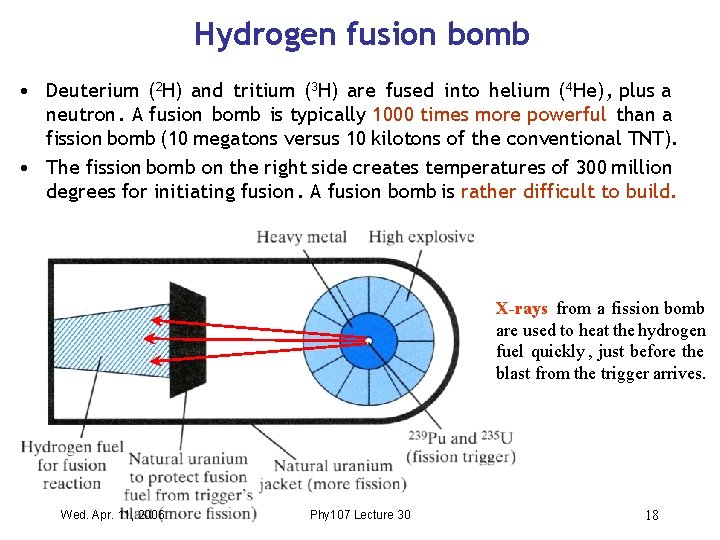
Hydrogen fusion bomb • Deuterium (2 H) and tritium (3 H) are fused into helium (4 He) , plus a neutron. A fusion bomb is typically 1000 times more powerful than a fission bomb (10 megatons versus 10 kilotons of the conventional TNT). • The fission bomb on the right side creates temperatures of 300 million degrees for initiating fusion. A fusion bomb is rather difficult to build. X-rays from a fission bomb are used to heat the hydrogen fuel quickly , just before the blast from the trigger arrives. Wed. Apr. 11, 2006 Phy 107 Lecture 30 18

The nuclear arsenal A small hydrogen bomb (1 megaton) Wed. Apr. 11, 2006 Lecture 30 19 H bombs inject soot into the Phy 107 stratosphere, causing nuclear winter.

Effect of a nuclear hit on San Francisco Hiroshima-size atomic bomb: Small hydrogen bomb: Downtown San Francisco is gone. All of San Francisco is gone. Blue: Destroyed by the blast. Red: Destroyed by fire. Wed. Apr. 11, 2006 Lecture 30 From Richard Muller, Physics. Phy 107 and Technology for Future Presidents 20
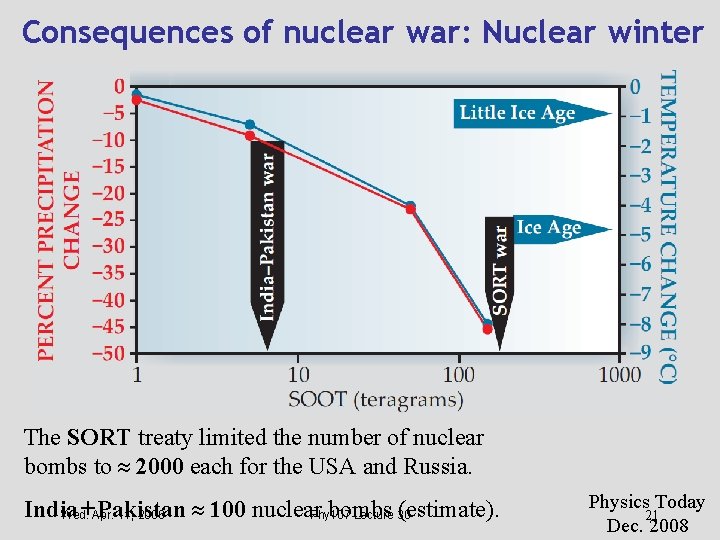
Consequences of nuclear war: Nuclear winter The SORT treaty limited the number of nuclear bombs to 2000 each for the USA and Russia. India +Apr. Pakistan 100 nuclear bombs (estimate). Wed. 11, 2006 Phy 107 Lecture 30 Physics Today 21 Dec. 2008
![Consequences of nuclear war: Failing crops [%] Reduction of the crop growing season in Consequences of nuclear war: Failing crops [%] Reduction of the crop growing season in](http://slidetodoc.com/presentation_image/0b340ff708de6a0d9cc0383abef6ab37/image-22.jpg)
Consequences of nuclear war: Failing crops [%] Reduction of the crop growing season in the 2 nd year after nuclear war. 11, 2006 Lecture 30 reduction lasts several years. 22 The. Wed. 1 st. Apr. year is a total wipeout. Phy 107 And the
Consequences of nuclear war: Summary The consequences of an all-out nuclear war go far beyond direct casualties (all major cities obliterated instantly). There is a worldwide breakdown of the infrastructure: • No crops for several years • No electricity, no fuel • No transportation for supplies • No factories to rebuild the infrastructure Wed. Apr. 11, 2006 Phy 107 Lecture 30 23
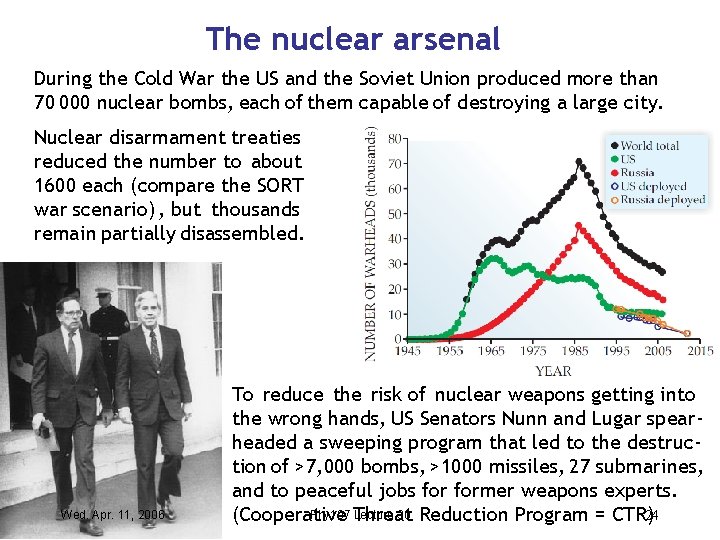
The nuclear arsenal During the Cold War the US and the Soviet Union produced more than 70 000 nuclear bombs, each of them capable of destroying a large city. Nuclear disarmament treaties reduced the number to about 1600 each (compare the SORT war scenario) , but thousands remain partially disassembled. Wed. Apr. 11, 2006 To reduce the risk of nuclear weapons getting into the wrong hands, US Senators Nunn and Lugar spearheaded a sweeping program that led to the destruction of >7, 000 bombs, >1000 missiles, 27 submarines, and to peaceful jobs former weapons experts. Phy 107 Threat Lecture 30 Reduction Program = CTR) 24 (Cooperative
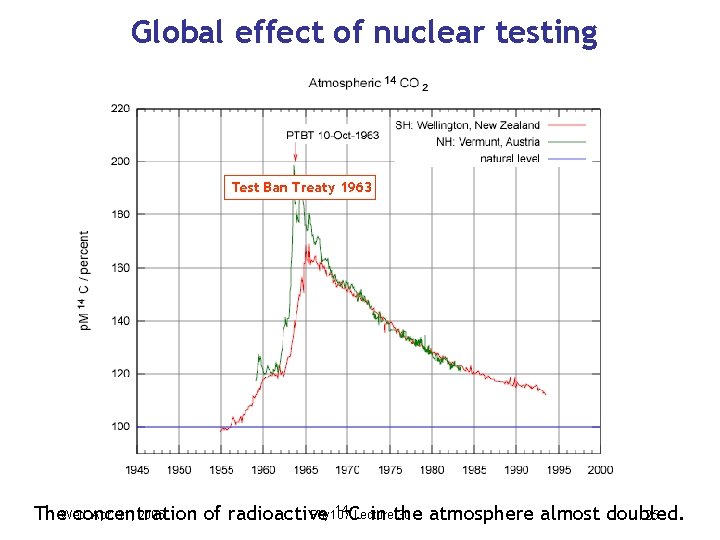
Global effect of nuclear testing Test Ban Treaty 1963 14 CLecture Apr. 11, 2006 Phy 107 30 The. Wed. concentration of radioactive in the atmosphere almost doubled. 25

Nuclear proliferation / terrorism Could a rogue nation or a terrorist group obtain nuclear weapons ? The main obstacle is either the enrichment of 235 U or a contained implosion of plutonium. H-bombs are much more difficult to build. Possible scenarios: • Seizing a bomb: It would be hard to find out how to explode the bomb. That would require inside information about the mechanism and code. • Seizing bomb material: The critical mass is about 25 kg 235 U, 8 kg 239 Pu. There are recipes on the internet how to make a nuclear bomb, but the infrastructure for handling these materials is difficult to assemble. • Sabotage of a nuclear power plant: The safety systems are designed such that a reactor automatically shuts down if anything goes wrong. It would require insider assistance to override the safety system. That happened at Chernobyl. • A dirty bomb (conventional explosive laced with radioactive material): It is a more likely but less damaging scenario. The damage would not spread far, but the psychological and economic fallout could be large. Wed. Apr. 11, 2006 Phy 107 Lecture 30 26
Nuclear Waste Fuel rods are usually discarded when about ¼ of the 4% enriched 235 U is consumed. That leaves 99% of the uranium as nuclear waste (mostly 238 U). Used fuel rods are kept in a cooling pool to allow the highly-radioactive nuclei to decay. At the Fukushima nuclear power plant, the pool was on top of each reactor. The storage pools exploded when hydrogen gas was formed by the overheating reactors underneath and reacted with oxygen. One can reprocess reactor fuel to extract the unused 99% uranium, which is done in France. That reduces the nuclear waste. But there is a risk that plutonium gets into the wrong hands. Nuclear waste storage is a problem. It needs to be safe for a long time. The half-life of plutonium is 24 000 years. Wed. Apr. 11, 2006 Phy 107 Lecture 30 27
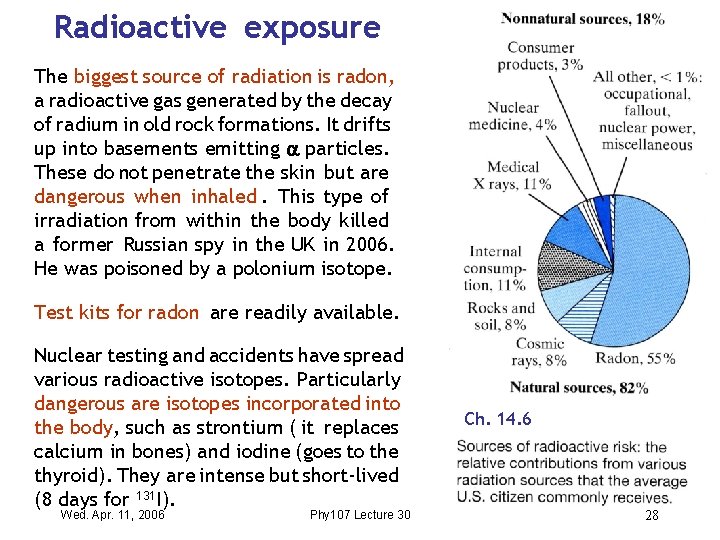
Radioactive exposure The biggest source of radiation is radon, a radioactive gas generated by the decay of radium in old rock formations. It drifts up into basements emitting particles. These do not penetrate the skin but are dangerous when inhaled. This type of irradiation from within the body killed a former Russian spy in the UK in 2006. He was poisoned by a polonium isotope. Test kits for radon are readily available. Nuclear testing and accidents have spread various radioactive isotopes. Particularly dangerous are isotopes incorporated into the body, such as strontium ( it replaces calcium in bones) and iodine (goes to the thyroid). They are intense but short-lived (8 days for 131 I). Wed. Apr. 11, 2006 Phy 107 Lecture 30 Ch. 14. 6 28
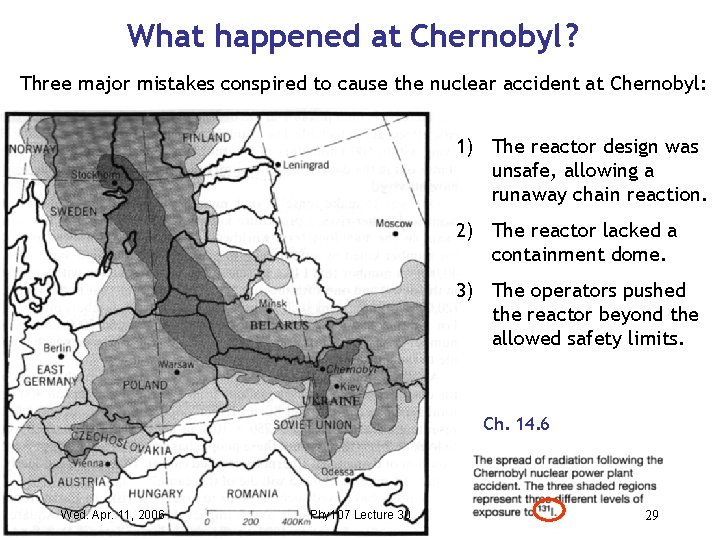
What happened at Chernobyl ? Three major mistakes conspired to cause the nuclear accident at Chernobyl: 1) The reactor design was unsafe, allowing a runaway chain reaction. 2) The reactor lacked a containment dome. 3) The operators pushed the reactor beyond the allowed safety limits. Ch. 14. 6 Wed. Apr. 11, 2006 Phy 107 Lecture 30 29
- Slides: 29