The Good the Bad And the Ugly Tales

The Good, the Bad, And the Ugly: Tales from the ELR Frontier Presented by Julia Knepp and Susan Neal-Lyman Johns Hopkins Medical Institutions

Disclaimers Presenter has no financial relationship with any commercial interest or conflicts of interest to this presentation. Setup and screenshots are from Soft. Lab. Mic version 4. 5. 4. 3. 8

The Good, the Bad, and the Ugly

Presentation Objectives • • MU 2 and ELR basics ELR required Soft Setup Issues Encountered ELR Solutions and Advice

The Lingo (Abbreviations) • DHMH – Maryland’s Department of Health and Mental Hygiene • CRISP – Chesapeake Regional Information Systems for our Patients (Maryland’s Health Information Exchange) • EHR – Electronic Health Record • ELR – Electronic Lab Reporting • HIE – Health Information Exchange • MU 2 – Meaningful Use 2 • NIST – National Institute of Standards and Technology • LOINC – Logical Observation Identifiers Names and Codes • RLR – Reportable Lab Results • SNOMED – Systematized Nomenclature of Medicine Clinical Terms

MU 2 and ELR Review

What is Meaningful Use? A Federal government incentive program that rewards providers and organizations that invest in Electronic Health Records (EHR) technology used by medical providers. – www. cdc. gov/EHRmeaningfuluse
ELR Benefits – MU 2 money – Reduced manual data entry errors – Improved TAT – No faxing errors – Reports that are more complete – Less trees www. cdc. gov/EHRmeaningfuluse
MU 2 – Panning for Gold The American Recovery and Reinvestment Act (ARRA) allocated “Approximately $160 billion to fund programs to improve and preserve health care, health information technology, children and community services, scientific research and facilities, and community health and prevention initiatives. ” http: //www. hhs. gov/recovery/ To obtain incentive payments, you must “attest” that the individual or organization is “meaningfully using the EHR”. www. fdclessons. com

Stage 2 Eligible Hospital Core Objective • • • (1) Use computerized provider order entry (CPOE) for medication, laboratory, and radiology orders directly entered by any licensed healthcare professional who can enter orders into the medical record per state, local, and professional guidelines. (2) Record all of the following demographics: preferred language, sex, race, ethnicity, date of birth, date and preliminary cause of death in the event of mortality in the eligible hospital or CAH. (3) Record and chart changes in the following vital signs: height/length and weight (no age limit); blood pressure (ages 3 and over); calculate and display body mass index (BMI); and plot and display growth charts for patients 0 -20 years, including BMI. (4) Record smoking status for patients 13 years old or older. (5) Use clinical decision support to improve performance on high-priority health conditions. (6) Provide patients the ability to view online, download, and transmit information about a hospital admission. (7) Protect electronic health information created or maintained by the Certified EHR Technology through the implementation of appropriate technical capabilities. • (8) Incorporate clinical lab test results into Certified EHR Technology as structured data. • • • (9) Generate lists of patients by specific conditions to use for quality improvement, reduction of disparities, research, or outreach. (10) Use clinically relevant information from Certified EHR Technology to identify patient-specific education resources and provide those resources to the patient. (11) The eligible hospital or CAH who receives a patient from another setting of care or provider of care or believes an encounter is relevant should perform medication reconciliation. • (12) The eligible hospital or CAH who transitions their patient to another setting of care or provider of care or refers their patient to another provider of care provides a summary care record for each transition of care or referral. • (13) Capability to submit electronic data to immunization registries or immunization information systems except where prohibited, and in accordance with applicable law and practice. • (14) Capability to submit electronic reportable laboratory results to public health agencies, where except where prohibited, and in accordance with applicable law and practice. • • (15) Capability to submit electronic syndromic surveillance data to public health agencies, except where prohibited, and in accordance with applicable law and practice. (16) Automatically track medications from order to administration using assistive technologies in conjunction with an electronic medication administration record (e. MAR). • https: //www. cms. gov/Regulations-and. Guidance/Legislation/EHRIncentive. Programs/Downloads/Stage 2_Meaningful. Use. Spec. Sheet_Table. Contents_Eligible. Hospitals_CAHs. pdf • • (14) Capability to submit electronic reportable laboratory results to public health agencies, where except where prohibited, and in accordance with applicable law and practice.

What is Electronic Lab Reporting? ELR is the electronic transmission from laboratories to public health of laboratory reports which identify reportable conditions. http: //www. cdc. gov/ehrmeaningfuluse/elr. html

Soft. Lab. Mic ELR Setup
ELR Test Setup A new ELR-specific test is required for each reportable result Insert New Record function in Soft. Lab Copy original test > Create New Test ID Providers ELR
ELR Test Setup shared with original test – LOINC – Containers – Values – Age Ranges No HIS Setup Table needed
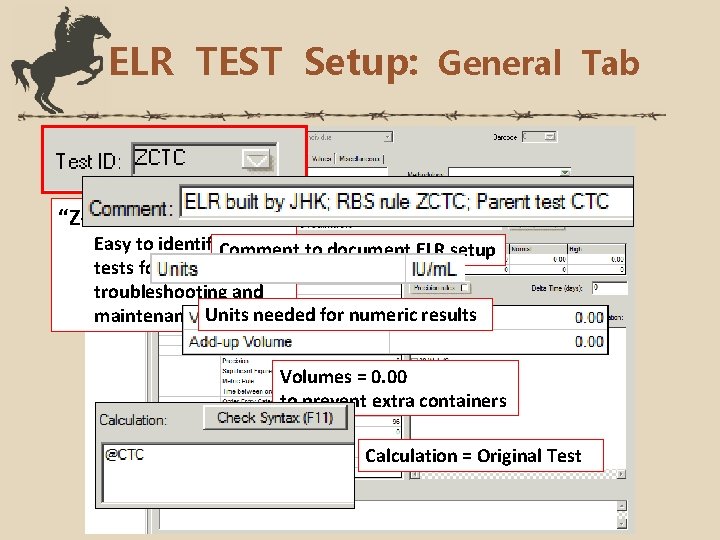
ELR TEST Setup: General Tab “Z-tests” Easy to identify. Comment as ELR to document ELR setup tests for future troubleshooting and maintenance Units needed for numeric results Volumes = 0. 00 to prevent extra containers Calculation = Original Test
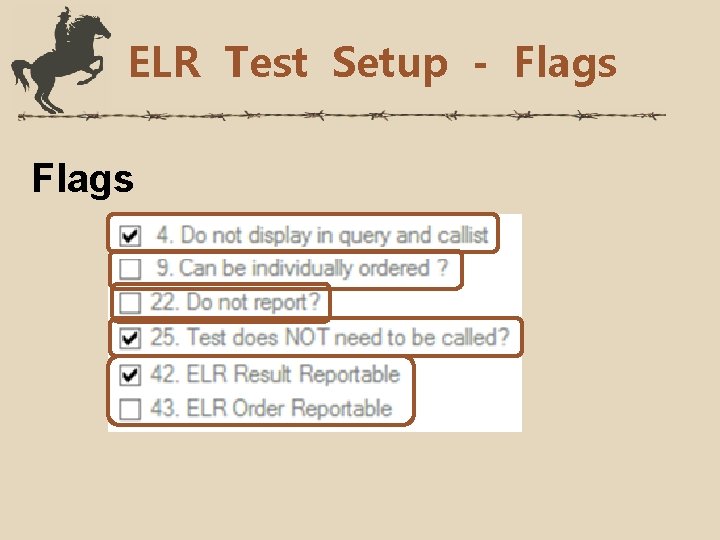
ELR Test Setup - Flags
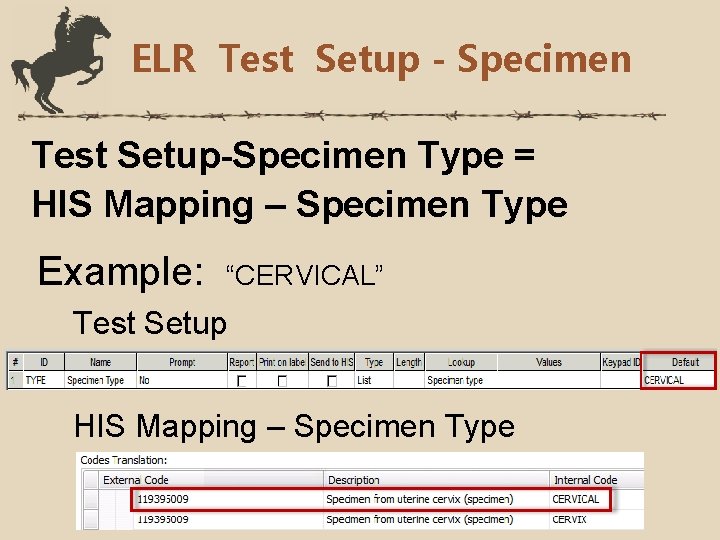
ELR Test Setup - Specimen Test Setup-Specimen Type = HIS Mapping – Specimen Type Example: “CERVICAL” Test Setup HIS Mapping – Specimen Type
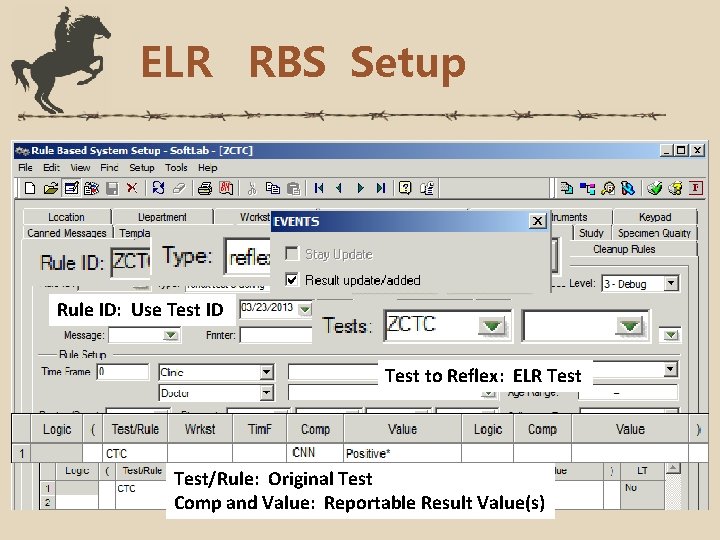
ELR RBS Setup Rule ID: Use Test ID Test to Reflex: ELR Test/Rule: Original Test Comp and Value: Reportable Result Value(s)
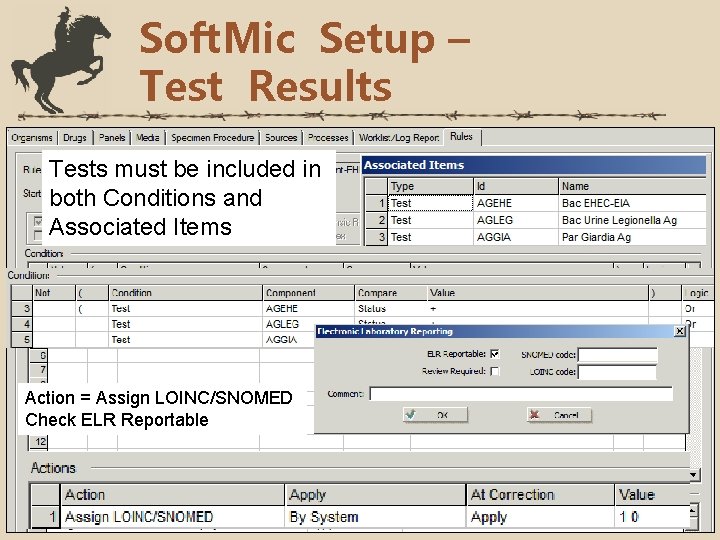
Soft. Mic Setup – Test Results Tests must be included in both Conditions and Associated Items Action = Assign LOINC/SNOMED Check ELR Reportable
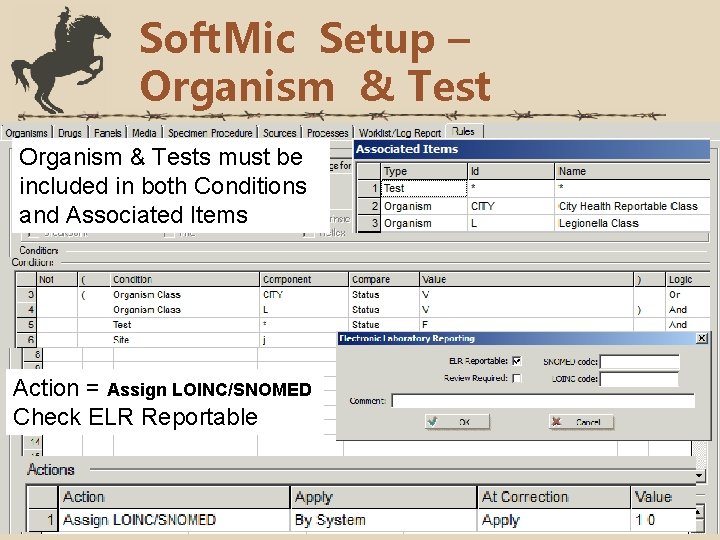
Soft. Mic Setup – Organism & Tests must be included in both Conditions and Associated Items Action = Assign LOINC/SNOMED Check ELR Reportable
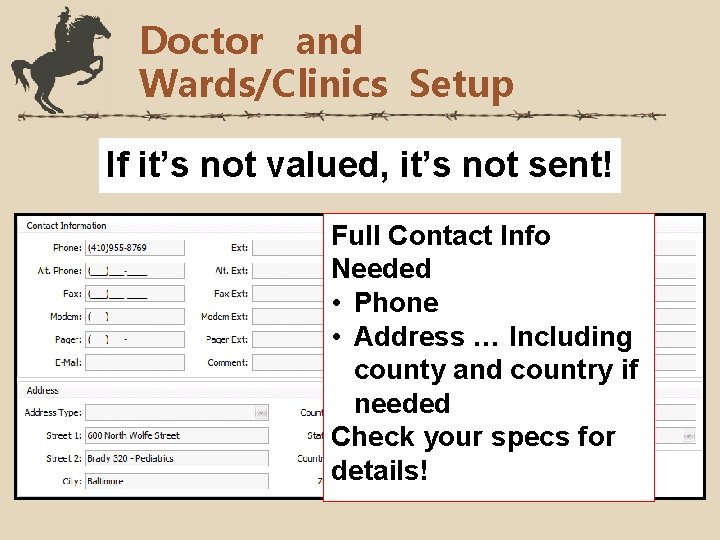
Doctor and Wards/Clinics Setup If it’s not valued, it’s not sent! Full Contact Info Needed • Phone • Address … Including county and country if needed Check your specs for details!
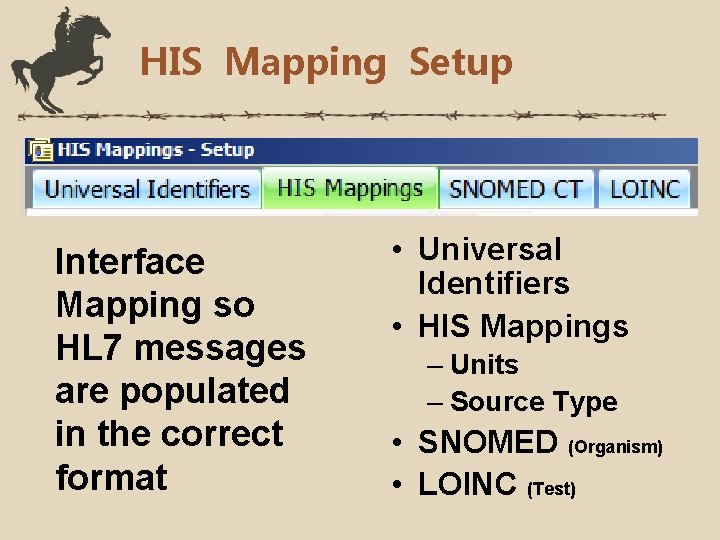
HIS Mapping Setup Interface Mapping so HL 7 messages are populated in the correct format • Universal Identifiers • HIS Mappings – Units – Source Type • SNOMED (Organism) • LOINC (Test)

Showdown at the ELR Spec Corral

HL 7 ELR Specs http: //www. hl 7. org/implement/standards/index. cfm

ADT Showdown If it’s not valued, then it’s not sent! How to get the data you need … imgbuddy. com

Lab vs. Epic Patient demographics incomplete or missing, but required for ELR Solution High level administrative talks and negotiations Epic takes responsibility for sending us the information we need

Lab vs. Affiliate Hospital Patient’s Ethnicity not sent to Soft Solution High level administrative talks and negotiations Affiliate Hospital agrees to send patient’s Ethnicity

Lab vs. Contract Clinics Patient demographics incomplete or missing, but required for ELR • Patients don’t supply information • Clinic HIS doesn’t have all required fields Solution Exclude the problem clinics from the RBS ELR reflex rules Continue faxing reporting; No ELR feed

Spec Validation Showdown A weary process of validation that reveals problems you didn’t even know you had …. www. dailymail. co. uk
SCC vs. State Health Department A classic case of He said, She said www. artic. edu
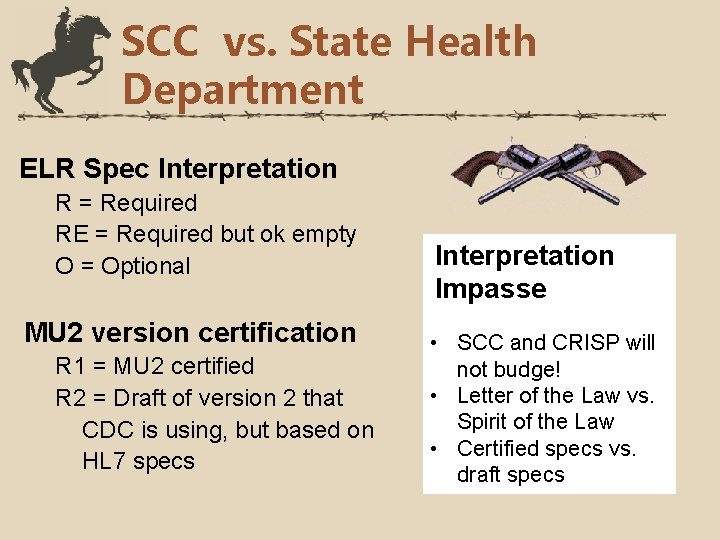
SCC vs. State Health Department ELR Spec Interpretation R = Required RE = Required but ok empty O = Optional MU 2 version certification R 1 = MU 2 certified R 2 = Draft of version 2 that CDC is using, but based on HL 7 specs Interpretation Impasse • SCC and CRISP will not budge! • Letter of the Law vs. Spirit of the Law • Certified specs vs. draft specs

Parent-Child Showdown Parent-Child Messages Micro result messages routinely have parent-child tests info. Most Lab result messages have one result. Lab reflex result messages also contain parent-child info. ginoruberto. 5 u. com
HIS Mappings Showdown HIS Mappings Setup is shared by ALL interfaces. Sometimes HIS interfaces need one mapping, but ELR interface needs it another. www. sistersinwonderland. eu

SCC Solutions Multiple Approaches 1. Data Stuffing into Setup Field 2. Hot Fixes 3. SCR www. laweekly. com

SCC Solutions When SCC can’t make changes, And you’ve reached the end of the line … en. wikipedia. org

Engine Solutions Your hospital’s interface engine is your lifesaver! The engine can … • Block certain segments • Convert standard text to specialized text www. awesomestories. com

Lessons Learned Advice from the ELR Frontier www. soundtrackcollector. com

The LOINC Roundup Start the LOINC coding process NOW! Hundreds, or even thousands, of tests will need the LOINC added to test setup Don’t forget to SNOMED code organisms, too! thesouthwestjournal. wordpress. com

Hospital Roundup Get buy in from your HIS colleagues, both on -site and at affiliate hospitals. To be MU 2 compliant, ALL agencies must take responsibility for their part.

ELR Specs – Taming the Process ELR Message Details Negotiate the details down to the mindnumbing minutia Flush Out the Problems Test early, test often, test all variations of patients, locations, and results Legends. Of. America. com

Westward Expansion: Multi-site Multi-state ELR Reporting www. legacystories. org
Reporting Requirements Where to send reportable results? Multisite Build • Main Campus – Maryland • Multi-site Affiliate Hospitals Maryland Washington DC
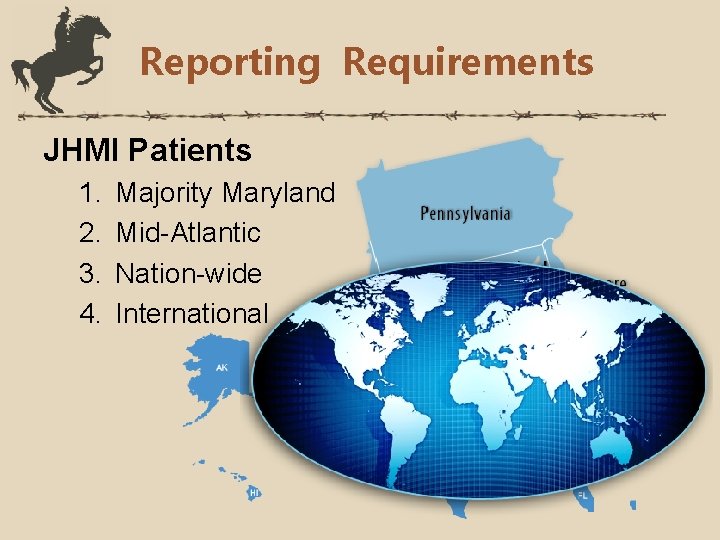
Reporting Requirements JHMI Patients 1. 2. 3. 4. Majority Maryland Mid-Atlantic Nation-wide International
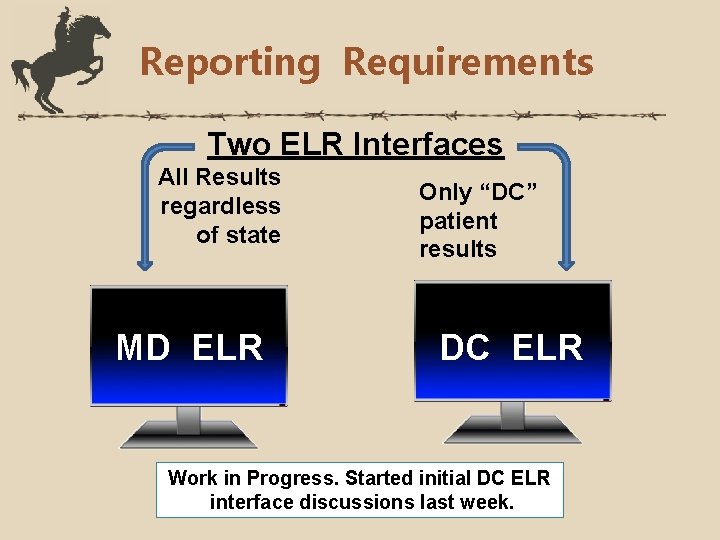
Reporting Requirements Two ELR Interfaces All Results regardless of state MD ELR Only “DC” patient results DC ELR Work in Progress. Started initial DC ELR interface discussions last week.

Resources • National Institute of Standards and Technology (NIST) Meaningful Use Tools http: //healthcare. nist. gov/use_testing/tools. html • Center for Disease Control (CDC) – Meaningful Use (MU 2) http: //www. cdc. gov/ehrmeaningfuluse/ – Electronic Lab Reporting (ELR) http: //www. cdc. gov/ehrmeaningfuluse/elr. html • Chesapeake Regional Information Systems for our Patients (CRISP) https: //crisphealth. org/ • SCC Implementers and Interface Specialists

In the End … Put your guns down, Clean them up, Rejoice in the fact that it’s over! imgkid. com www. pinterest. com
Good Luck!!!

Questions … ELR Fron tier
- Slides: 48