The GOLIATH Study The GOLIATH Study 1 2




- Slides: 10

The GOLIATH Study .

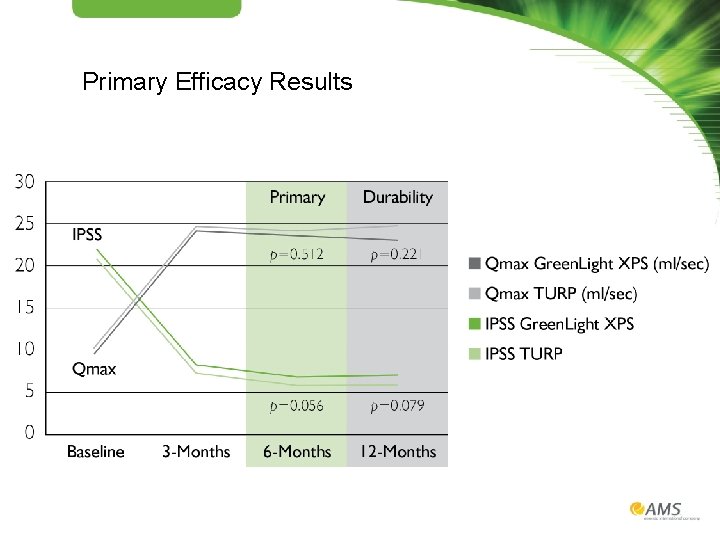
The GOLIATH Study 1, 2 • • • Prospective, randomized multicenter controlled trial 29 centers, 9 European countries First randomized controlled trial powered to compare the safety and efficacy of the 180 W Green. Light XPS™ Laser Therapy System with transurethral resection of the prostate (TURP) PRIMARY OBJECTIVES: SECONDARY OBJECTIVES: Non-inferiority of 180 W Green. Light XPS System compared with TURP • International Prostate Symptom Score (IPSS) at 6 months • Maximum flow rate (Qmax) at 6 months • Proportion of patients who are complication free through 180 days • • • Length of catheterization Length of hospitalization Time until stable health Post-void residual urine volume (PVR) Prostate volume (PV) Prostatic-specific antigen (PSA)

Primary Efficacy Results

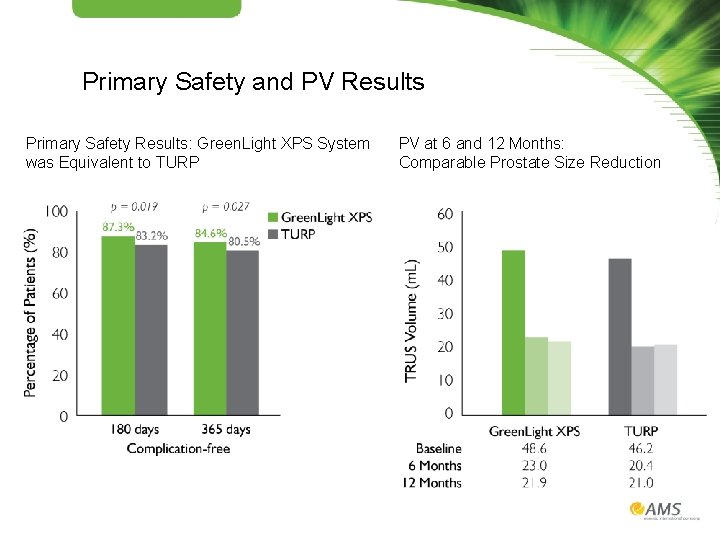
Primary Safety and PV Results Primary Safety Results: Green. Light XPS System was Equivalent to TURP PV at 6 and 12 Months: Comparable Prostate Size Reduction

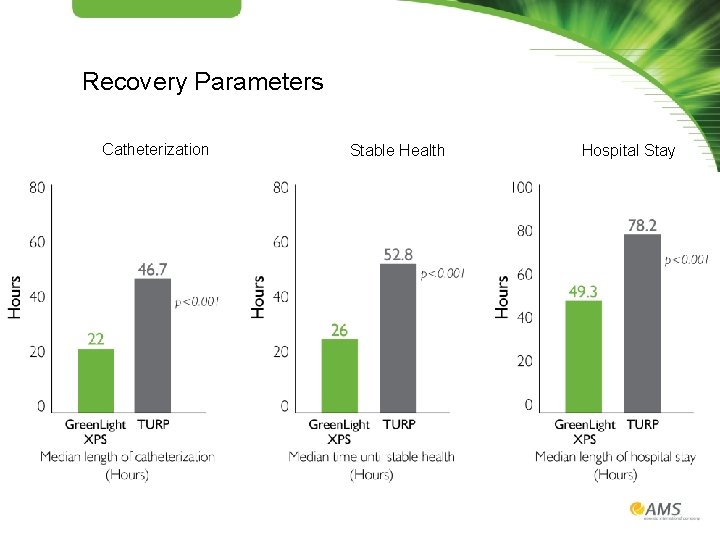
Recovery Parameters Catheterization Stable Health Hospital Stay

Adverse Events • No significant difference could be detected between the Green. Light XPS System and TURP with regards to any adverse events (AE) (p=0. 330) • Storage symptoms (dysuria/irritative pain/discomfort) were the most commonly observed Grade I events in 18. 4% (Green. Light XPS System) and 18. 0% (TURP) patients • Urinary tract infections (defined by intention to treat without microbiological confirmation documented) were the most commonly observed Grade II events in 16. 2% after the Green. Light XPS procedure and 9. 0% after TURP (p=0. 098) • Statistically significant difference in early AE (48 hours to 30 days) with 12 complications in the TURP group and zero in the Green. Light XPS procedure group (p=<0. 001) • At 12 months, ongoing self-reported urinary leakage of any degree was observed in 4 (2. 9%) after the Green. Light XPS procedure and 4 (3. 0%) after TURP; this has been easily tolerated without intervention

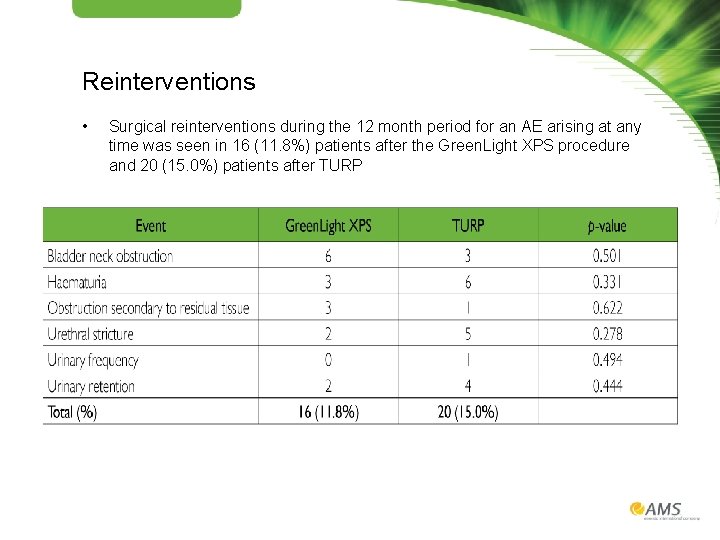
Reinterventions • Surgical reinterventions during the 12 month period for an AE arising at any time was seen in 16 (11. 8%) patients after the Green. Light XPS procedure and 20 (15. 0%) patients after TURP

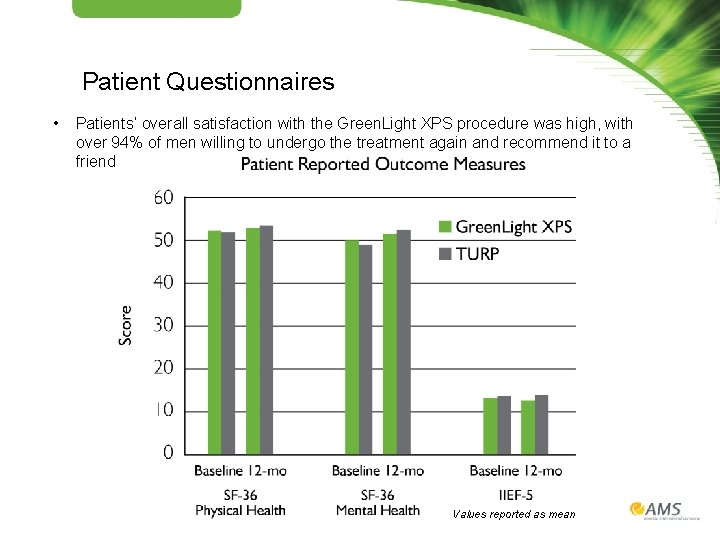
Patient Questionnaires • Patients’ overall satisfaction with the Green. Light XPS procedure was high, with over 94% of men willing to undergo the treatment again and recommend it to a friend Values reported as mean

Conclusions First randomized controlled trial powered to compare safety and efficacy of the Green. Light XPS Laser System and TURP demonstrated • TURP resulted in 5 times more surgical interventions to resolve post-operative bleeding than Green. Light XPS procedure • Comparable results in terms of IPSS, Qmax, and complication-free after 12 months • Patients treated with the Green. Light XPS System had a significantly shorter median length of catheterization, time until stable health, and hospitalization compared with TURP • Overall portion of patients free from any AE was comparable between the Green. Light XPS procedure and TURP • In the 48 hour to 30 day period, there were 0 AE in the Green. Light XPS System arm compared to 12 in the TURP arm (p<0. 001) • Comparable storage symptoms (dysuric or irritative symptoms) between treatment arms • At 12 months, self reported urinary leakage of any degree was reported in 2. 9% of the Green. Light XPS System procedure patients and 3% of TURP patients • Overall post-operative re-intervention rates were not significantly different between treatment arms

Brief Summary The Green. Light™ laser system is intended for incision/excision, vaporization, ablation, hemostasis and coagulation of soft tissue, including photoselective vaporization of the prostate for benign prostatic hyperplasia (BPH). The laser system is contraindicated for patients who: are contraindicated for surgery, contraindicated where appropriate anesthesia is contraindicated by patient history, have calcified tissue, require hemostasis in >2 mm vessels, have uncontrolled bleeding disorders, have prostate cancer, have acute urinary tract infection (UTI) or severe urethral stricture. Possible risks and complications include, but are not limited to, irritative symptoms (dysuria, urgency, frequency), retrograde ejaculation, urinary incontinence, erectile dysfunction, hematuria - gross, UTI, bladder neck contracture/outlet obstruct, urinary retention, perforation - prostate, urethral stricture. Prior to using these devices, please review the Operator’s Manual and any accompanying instructions for use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events. 1. 2. Bachmann A, Tubaro A, Barber N, et al. , 180 -W XPS Green. Light Laser Vaporization Versus Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: 6 -Month Safety and Efficacy Results of a European Multicentre Randomized Trial—The GOLIATH Study; European Urology, 2014 May; 65(5): 931 -42 Bachmann A, Tubaro A, Barber N, et al. , An European Multicenter Randomized Noninferiority Trial Comparing 180 -W Green. Light-XPS Laser Vaporization and Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: 12 Month Results of the GOLIATH-Study; Journal of Urology, 2014 Sep 11. pii: S 0022 -5347(14)04377 -8. doi: 10. 1016/j. juro. 2014. 09. 001. Rx Only ™ The denoted marks are trademarks or registered trademarks of American Medical Systems, Inc. © 2014 American Medical Systems, Inc. All Rights Reserved. Minnetonka, MN 55343 AMSUS/GL-00911 b(2)/September 2014 www. American. Medical. Systems. com 1 -800 -328 -3881 U. S. and International Use