The General Adaptation Syndrome Figure 18 21 Flowsheet







- Slides: 25

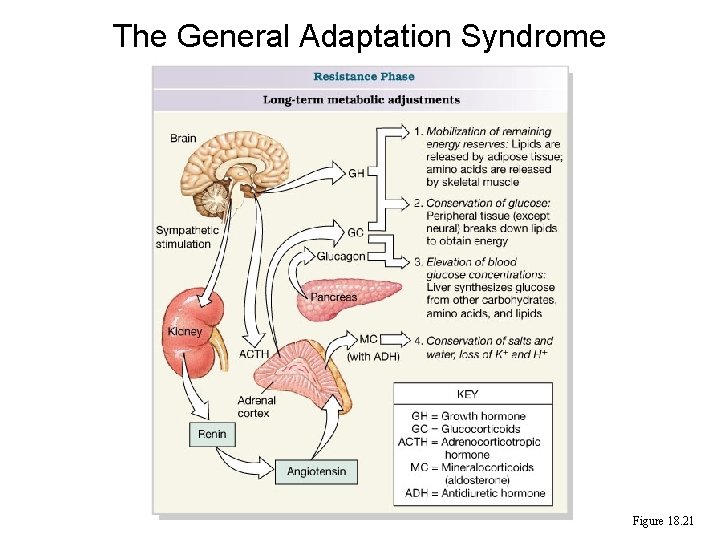
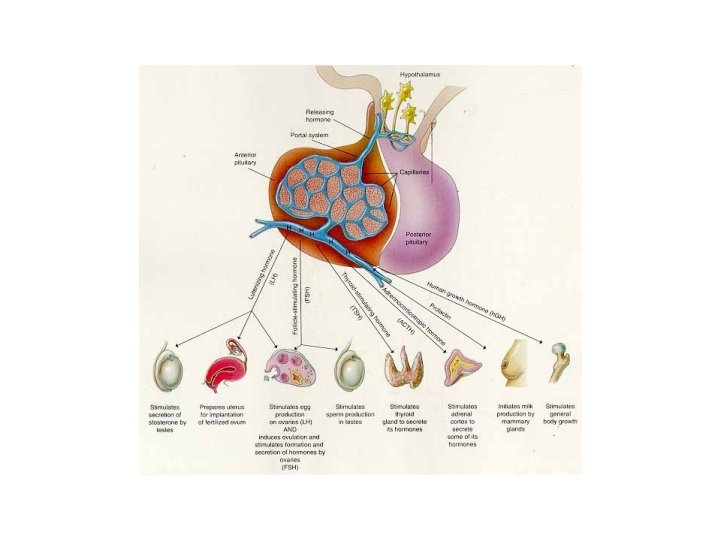
The General Adaptation Syndrome Figure 18. 21

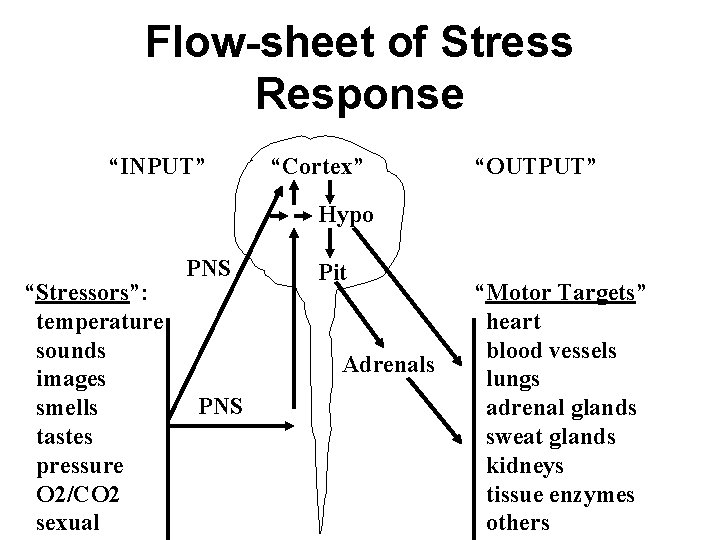
Flow-sheet of Stress Response “INPUT” “Cortex” “OUTPUT” Hypo “Stressors”: temperature sounds images smells tastes pressure O 2/CO 2 sexual PNS Pit Adrenals PNS “Motor Targets” heart blood vessels lungs adrenal glands sweat glands kidneys tissue enzymes others

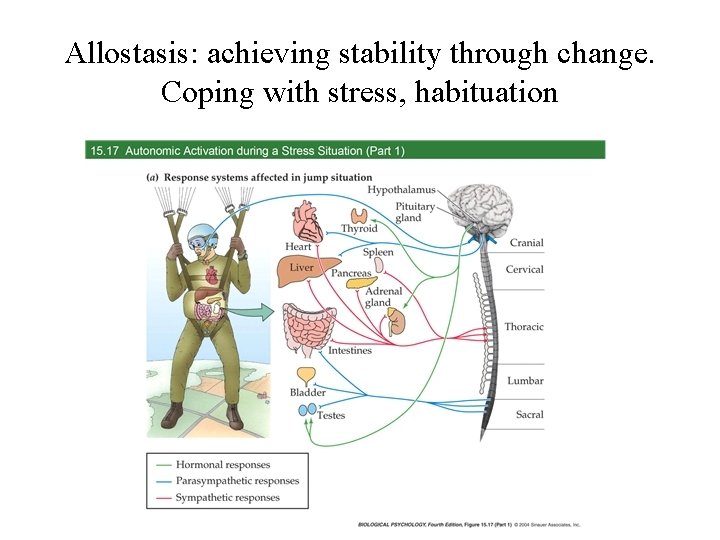
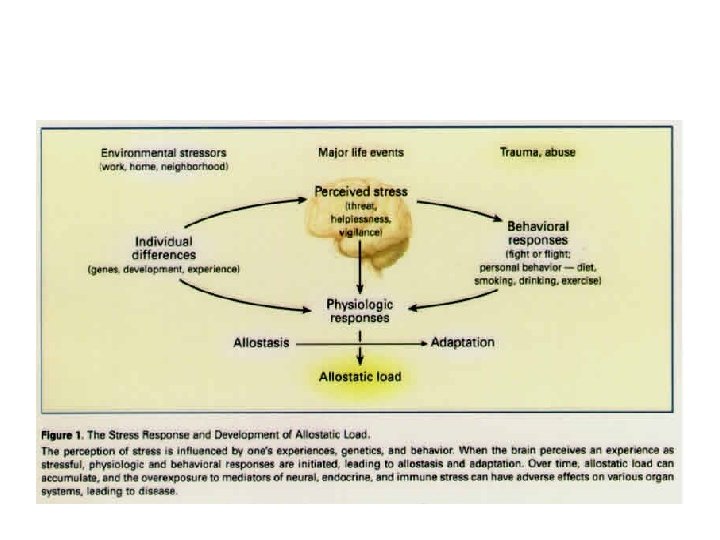
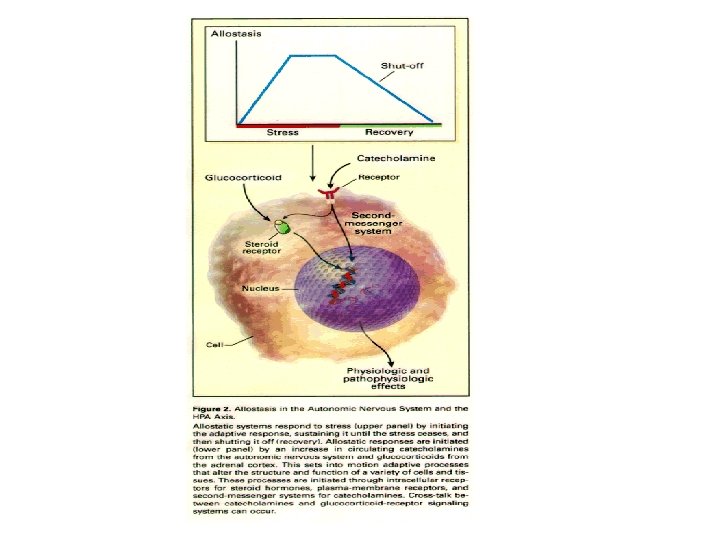
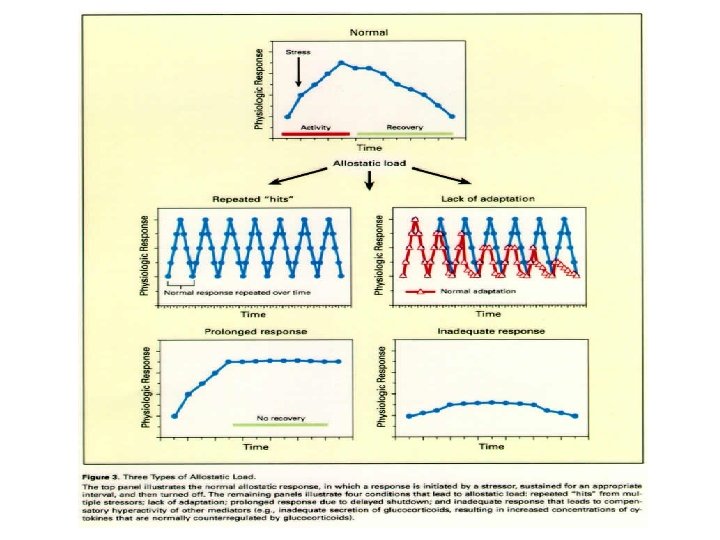
Allostasis: achieving stability through change. Coping with stress, habituation


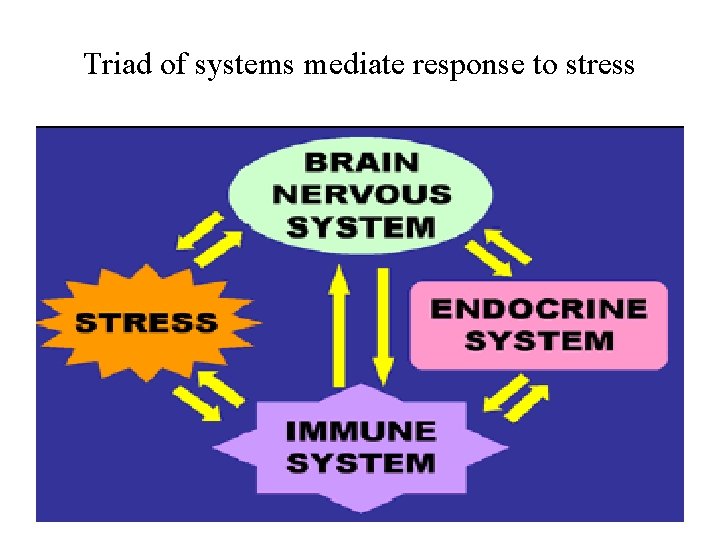
Triad of systems mediate response to stress

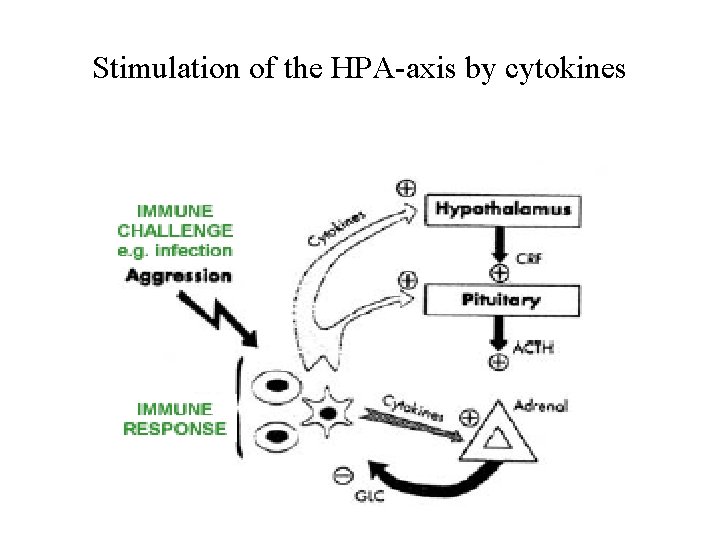
Stimulation of the HPA-axis by cytokines





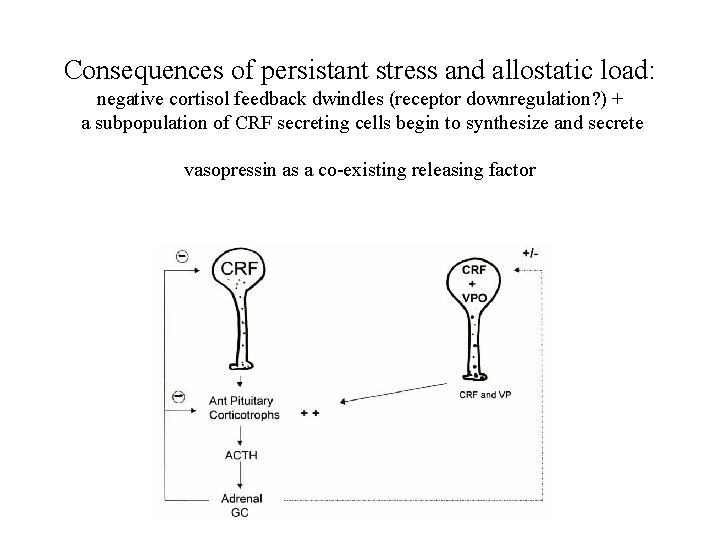
Consequences of persistant stress and allostatic load: negative cortisol feedback dwindles (receptor downregulation? ) + a subpopulation of CRF secreting cells begin to synthesize and secrete vasopressin as a co-existing releasing factor

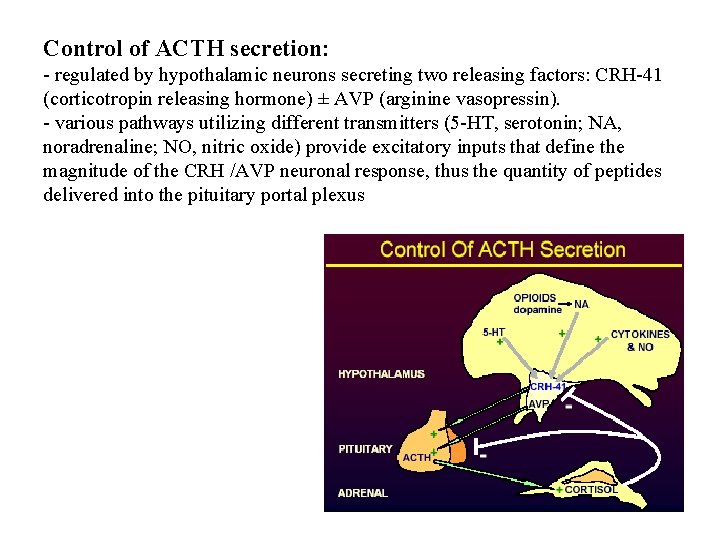
Control of ACTH secretion: - regulated by hypothalamic neurons secreting two releasing factors: CRH-41 (corticotropin releasing hormone) ± AVP (arginine vasopressin). - various pathways utilizing different transmitters (5 -HT, serotonin; NA, noradrenaline; NO, nitric oxide) provide excitatory inputs that define the magnitude of the CRH /AVP neuronal response, thus the quantity of peptides delivered into the pituitary portal plexus


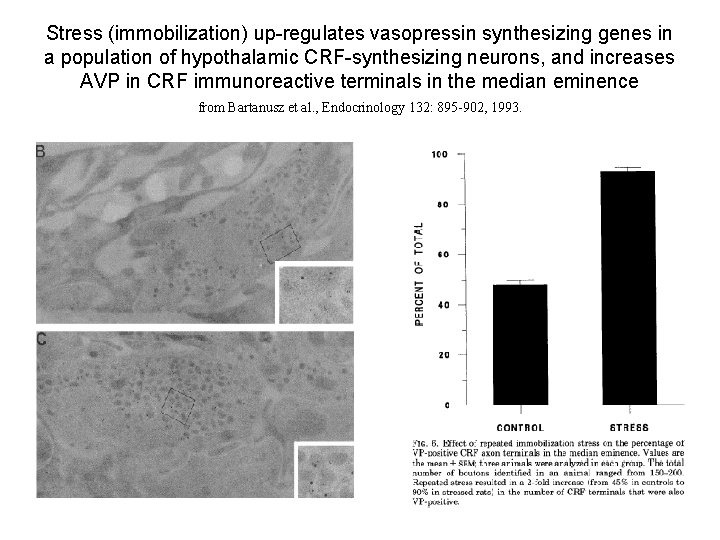
Stress (immobilization) up-regulates vasopressin synthesizing genes in a population of hypothalamic CRF-synthesizing neurons, and increases AVP in CRF immunoreactive terminals in the median eminence from Bartanusz et al. , Endocrinology 132: 895 -902, 1993.

Summary: Chronic stress changes induced in the hypothalamic CRF system • Prolonged exposure of the hippocampus to elevated cortisol levels impairs inhibitory feedback to CRF cells • Add: reductions in the impact of negative cortisol feedback, so CRF cells more active → more drive on HPA axis • Add: re-activation of vasopressin genes in a subpopulation of CRF cells, so they now produce two (stimulatory) peptides for secretion into the portal plexus • Add: upregulation of vasopressin V 1 b type receptors in anterior pituitary corticotrophs (double whammy → more ACTH→more drive on HPA axis) • Add: additional drive to PVN from other sites e. g. amygdala


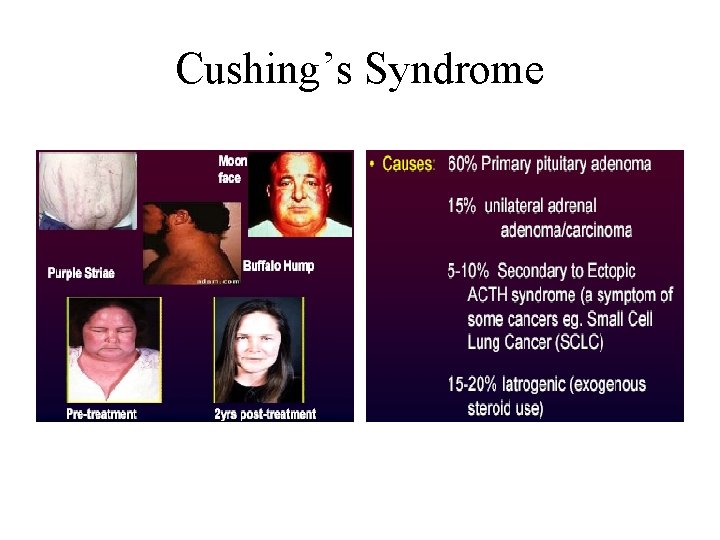
Cushing’s Syndrome

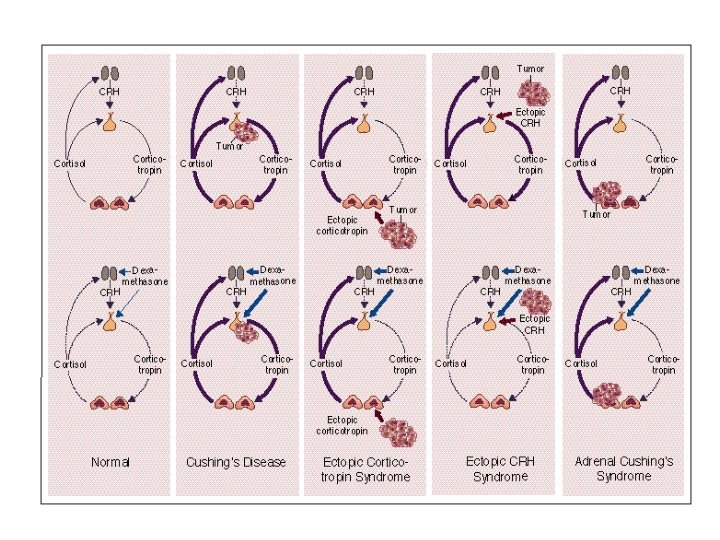
Cushing’s Syndrome DN Orth, NEJM 332: 791 -803, 1995 Figure 1. Physiologic and Pathophysiologic Features of the Hypothalamic– Pituitary–Adrenal Axis in Normal Subjects and Patients with Cushing's Syndrome (Top Panels) and the Effect of Dexamethasone (Bottom Panels). Stimulation of the hypothalamus by other central nervous system centers, such as the locus caeruleus, regulates the secretion of CRH; corticotropin stimulates adrenal secretion of cortisol; and cortisol inhibits the secretion of both CRH and corticotropin. Adrenal (i. e. , corticotropin-independent) Cushing's syndrome is caused by adrenal tumors and corticotropinindependent bilateral micronodular and macronodular adrenal hyperplasia. Low doses of dexamethasone are shown by thin blue arrows, and high doses by thick blue arrows. Normal hormone secretion is shown by thin purple lines, suppressed secretion by dotted purple lines, and hypersecretion by thick purple lines.





Neuron, Vol. 20, 1093– 1102, June, 1998, Copyright ã 1998 by Cell Press Corticotropin Releasing Factor Receptor 1–Deficient Mice Display Decreased Anxiety, Impaired Stress Response, and Aberrant Neuroendocrine Development Corticotropin releasing factor (CRF) is a major integrator of adaptive responses to stress. Two biochemically and pharmacologically distinct CRF receptor subtypes (CRFR 1 and CRFR 2) have been described. We have generated mice null for the CRFR 1 gene to elucidate the specific developmental and physiological roles of CRF receptor mediated pathways. Behavioral analyses revealed that mice lacking CRFR 1 displayed markedly reduced anxiety. Mutant mice also failed to exhibit the characteristic hormonal response to stress due to a disruption of the hypothalamicpituitary-adrenal (HPA) axis. Homozygous mutant mice derived from crossing heterozygotes displayed low plasma corticosterone concentrations resulting from a marked agenesis of the zona fasciculata region of the adrenal gland. The offspring from homozygote crosses died within 48 hr after birth due to a pronounced lung dysplasia. The adrenal agenesis in mutant animals was attributed to insufficient adrenocorticotropic hormone (ACTH) production during the neonatal period and was rescued by ACTH replacement. These results suggest that CRFR 1 plays an important role both in the development of a functional HPA axis and in mediating behavioral changes associated with anxiety.

