The Gaseous State Chapter 10 Kinetic Theory Kinetic











- Slides: 14

The Gaseous State Chapter 10

Kinetic Theory • Kinetic energy is the NRG of motion • The Kinetic Theory states that all particles of matter are in constant motion

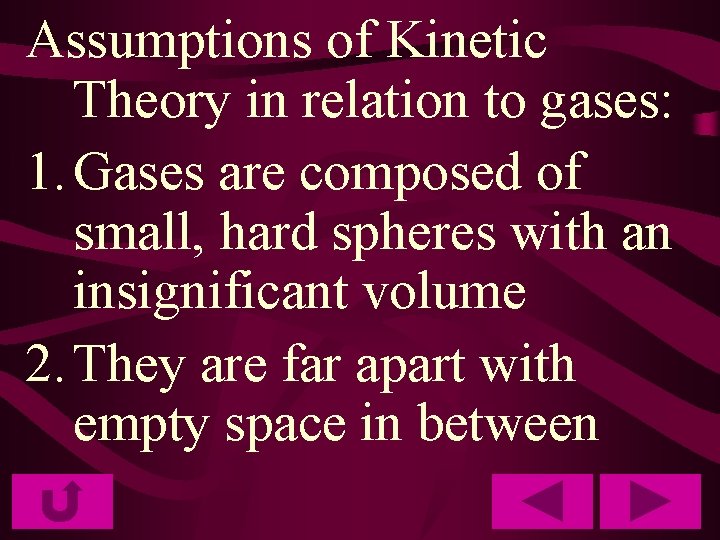
Assumptions of Kinetic Theory in relation to gases: 1. Gases are composed of small, hard spheres with an insignificant volume 2. They are far apart with empty space in between

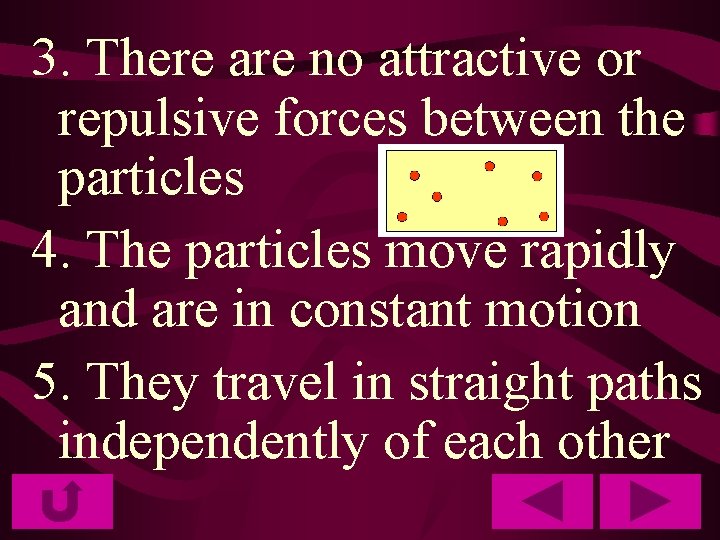
3. There are no attractive or repulsive forces between the particles 4. The particles move rapidly and are in constant motion 5. They travel in straight paths independently of each other

6. Gases fill their container indefinitely (they will expand) 7. Only change direction after colliding with something else 8. All collisions are perfectly elastic – no energy loss

• The kinetic theory is helpful in explaining gas pressure • Gas pressure: force exerted by a gas on its container • An empty space with no particles = vacuum = no pressure

• Atmospheric pressure – the pressure of air around earth – dependent on the weather • Barometers measure air pressure

• SI Unit of pressure is the pascal (Pa) • At sea level, Atm. Pressure is 101. 3 kilopascals (k. Pa) • mm. Hg and atmospheres (atm) are older units of pressure • 1 atm = 760 mm. Hg = 101. 3 k. Pa

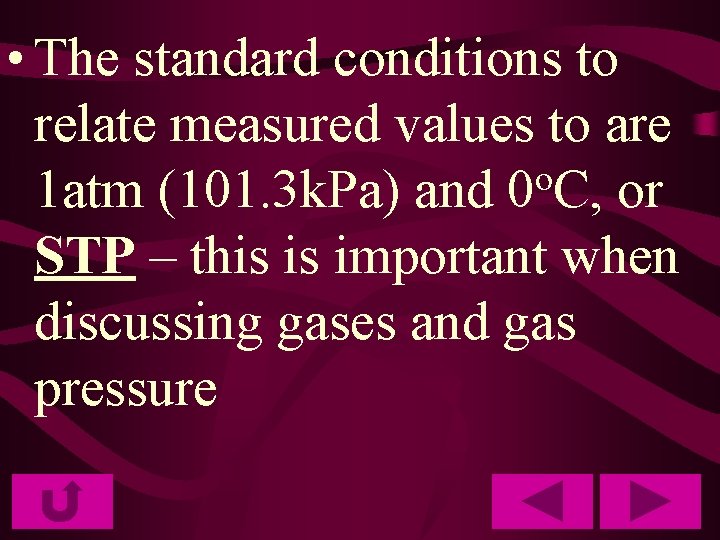
• The standard conditions to relate measured values to are o 1 atm (101. 3 k. Pa) and 0 C, or STP – this is important when discussing gases and gas pressure

• When a substance is heated, the particles store some of the absorbed NRG (potential) but the NRG not stored increases the speed of the particles, the average kinetic NRG & the temperature

• The actual particles have a wide range of kinetic energies – but temperature is the average kinetic energy • At high temperatures, there is a wider range of kinetic NRGs

• As a substance cools, the particles lose energy & slow down o • Absolute Zero (0 K, -273 C) theoretical temperature when no movement occurs = no NRG

• Check out this website tutorial on the States of Matter, from CHEMTUTOR. com Click HERE for Tutorial

• Now, move on to the Phase Changes Power Point Click Here to go on to the Phase Changes Power Point
 Gaseous state chapter
Gaseous state chapter My very excited mother planets
My very excited mother planets Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Gaseous exchange in protozoa
Gaseous exchange in protozoa Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Gaseous exchange in grasshopper
Gaseous exchange in grasshopper Granular porosity denture
Granular porosity denture Gaseous exchange in animals
Gaseous exchange in animals Inhalanda
Inhalanda Gaseous equilibrium
Gaseous equilibrium A thin gaseous layer that envelopes the earth
A thin gaseous layer that envelopes the earth Life science grade 11 gaseous exchange practical
Life science grade 11 gaseous exchange practical Pearson
Pearson Gas and liquid solution example
Gas and liquid solution example Khbo2
Khbo2