The Gas Phase Chapter 6 Properties of Gases




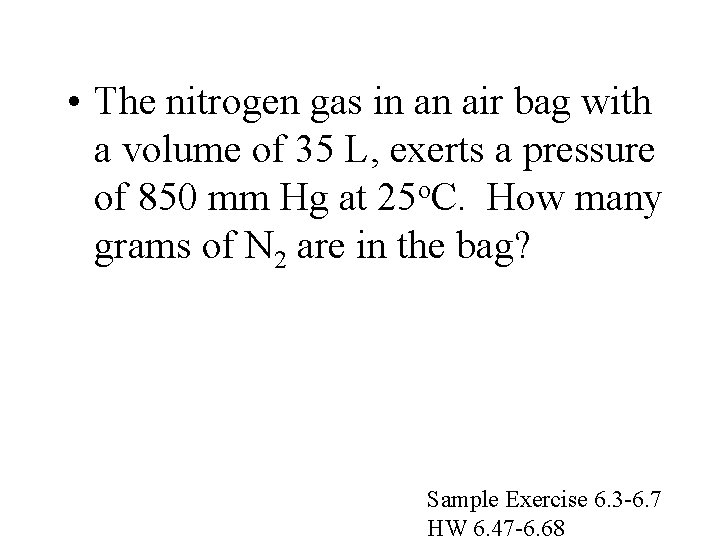

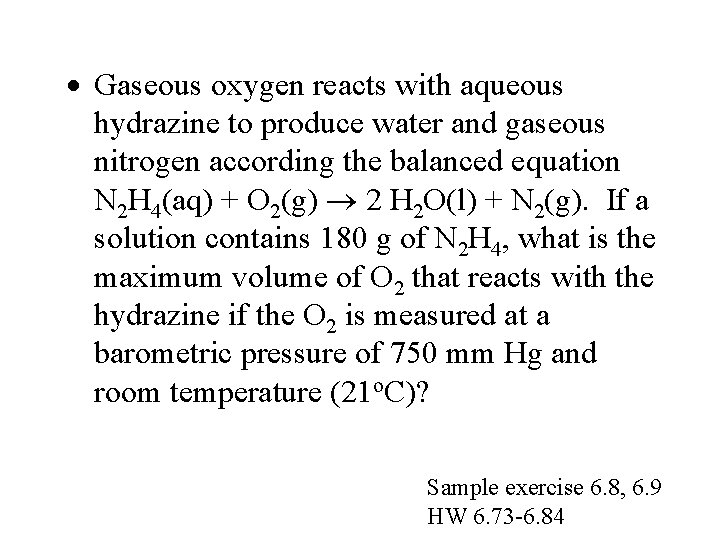







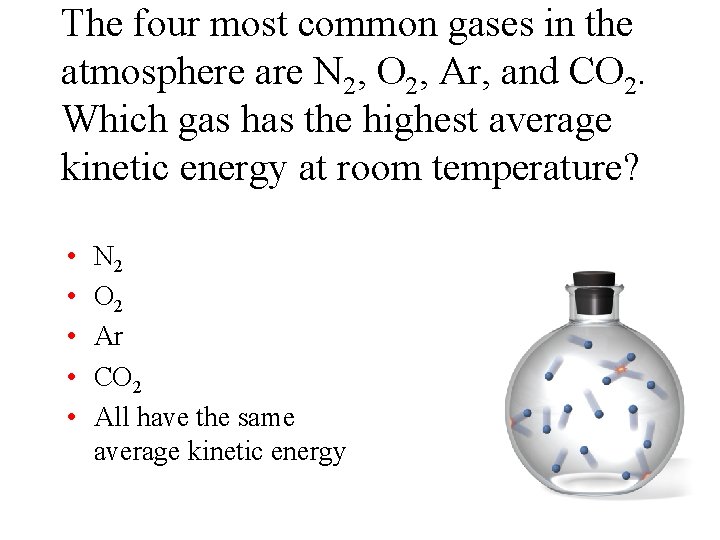

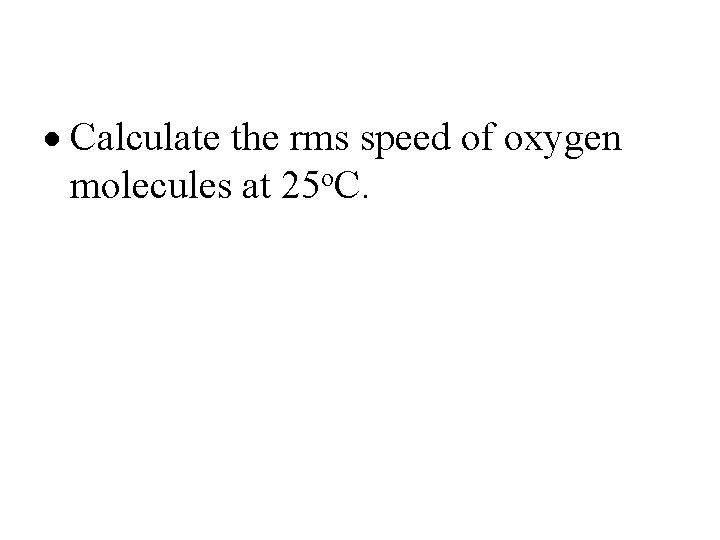





- Slides: 67

The Gas Phase Chapter 6

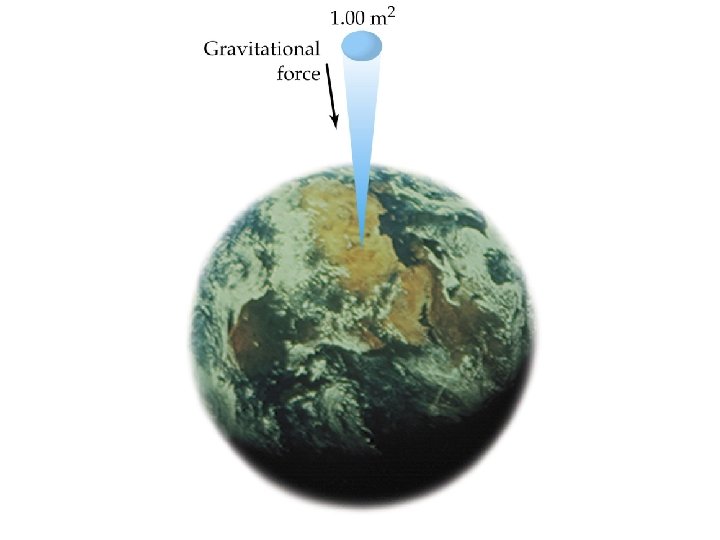
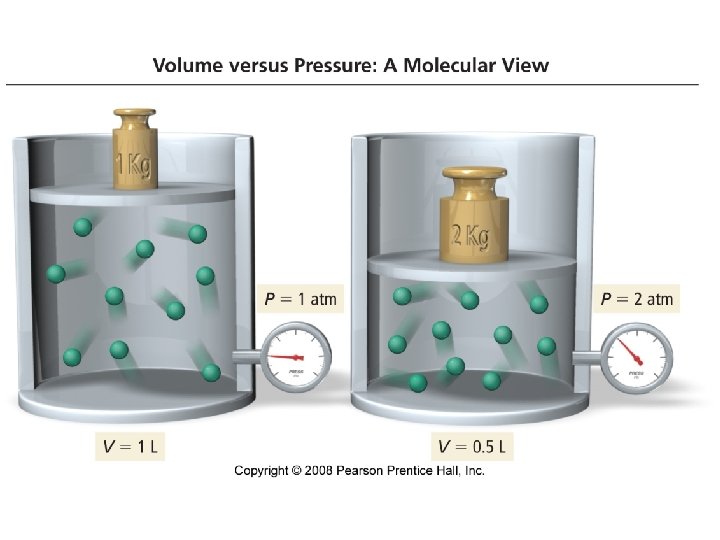
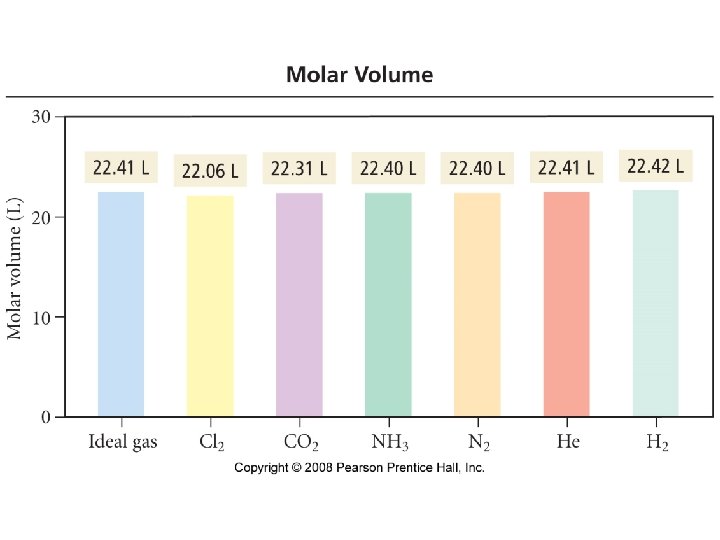
Properties of Gases • Gases form homogeneous mixtures • Gases are compressible • All gases have low densities · air · water 1. 00 · iron 7. 9 0. 0013 g/m. L • Gases expand to fill their containers uniformly • A gas exerts a pressure

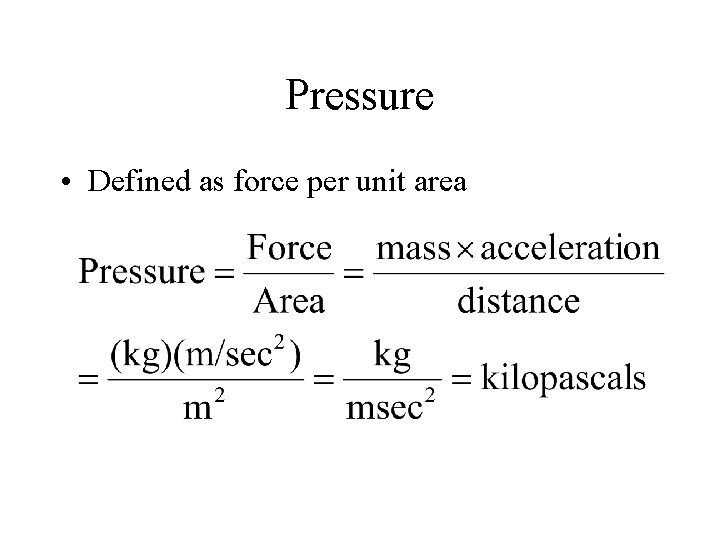
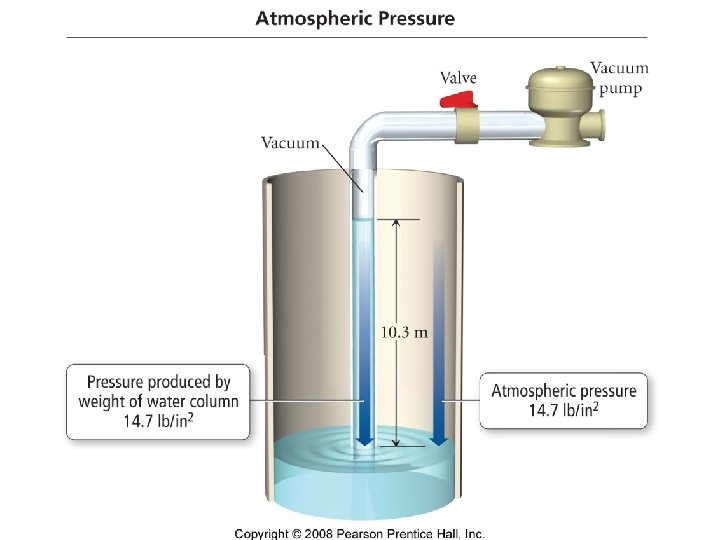
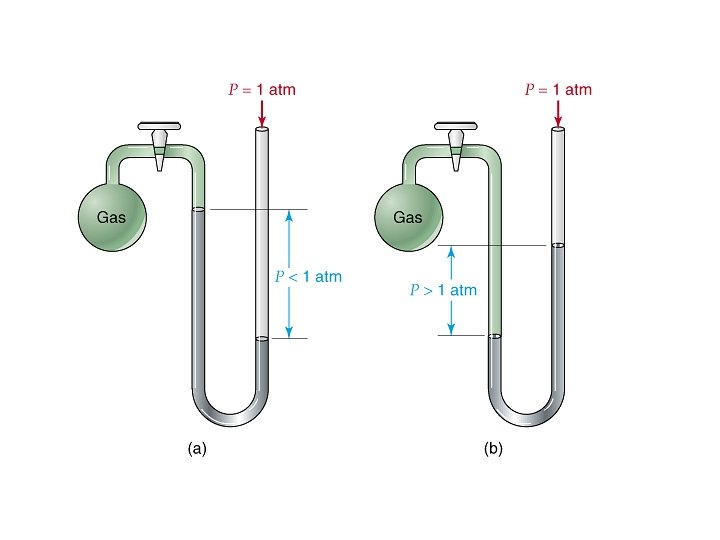
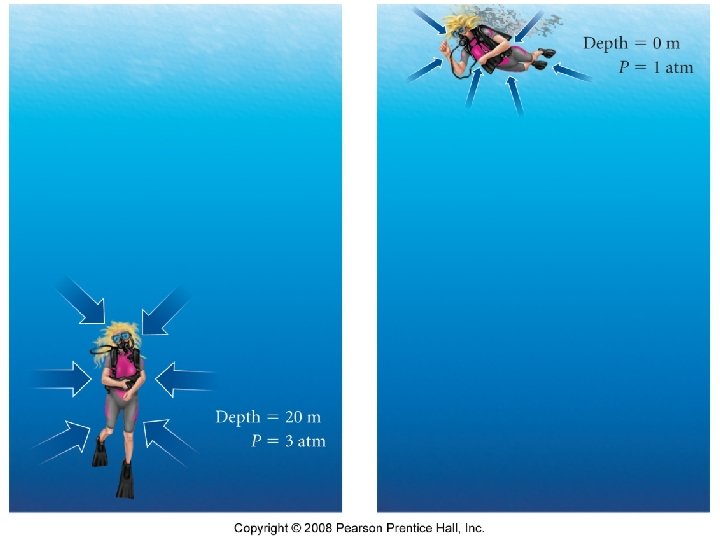
Pressure • Defined as force per unit area





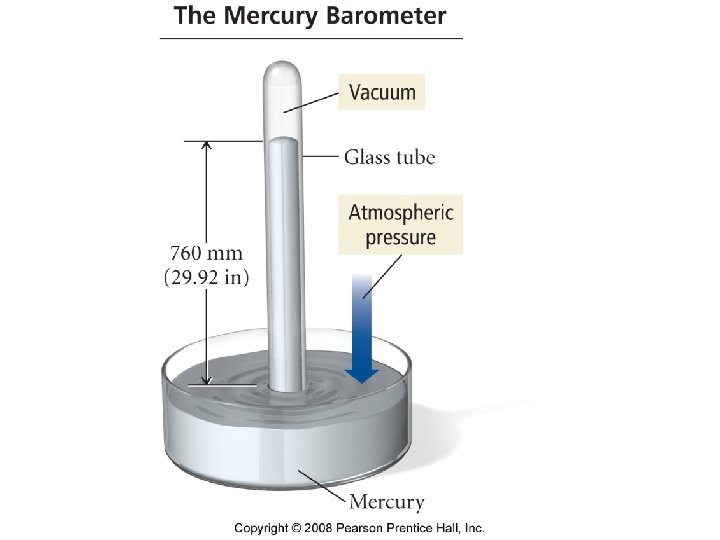
Pressure Units • • • 101. 325 k. Pa = 760 mm. Hg =760 torr =1 atm =30 in Hg =14. 7 psi






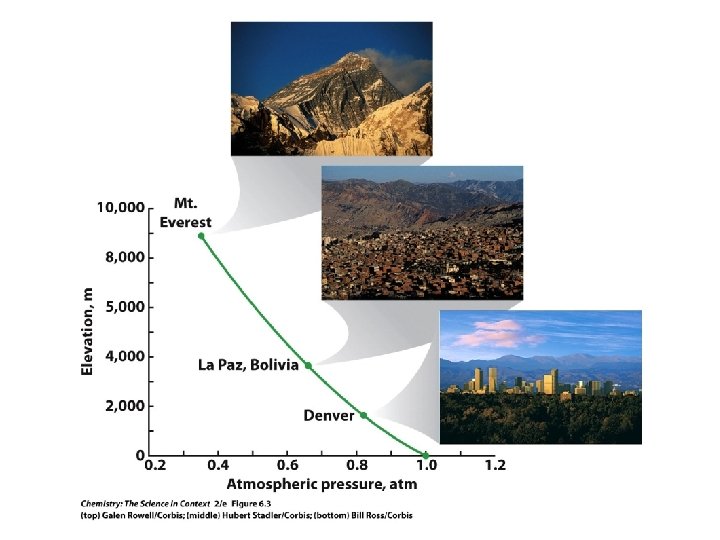
• The atmospheric pressure is 638 torr. What is the pressure in atmospheres? Sample Exercise 6. 1 HW 6. 31 -6. 40


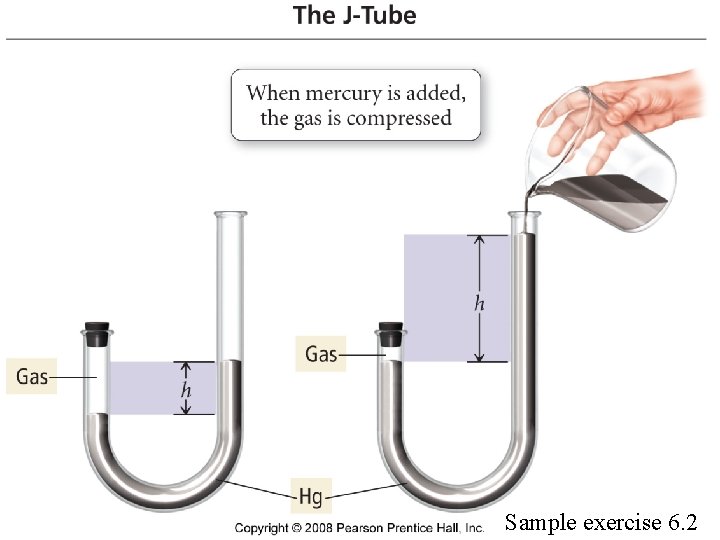
Sample exercise 6. 2




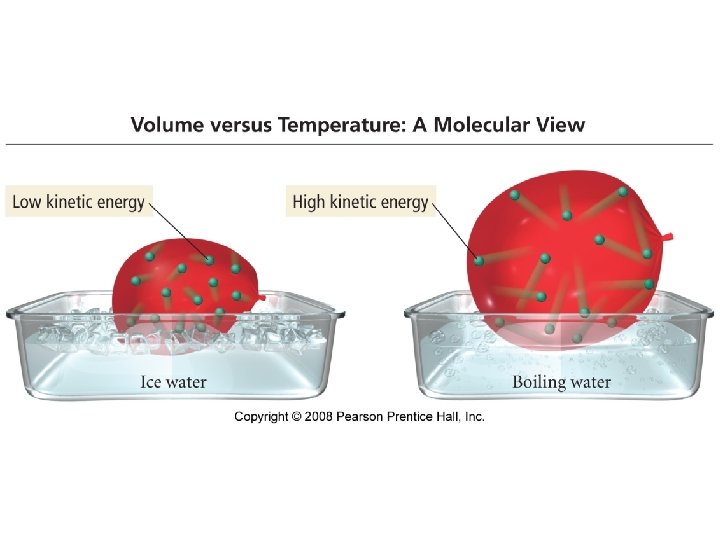
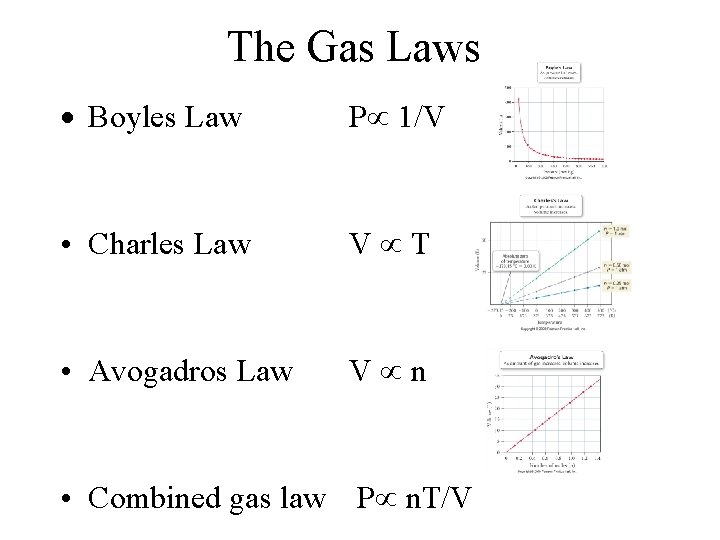
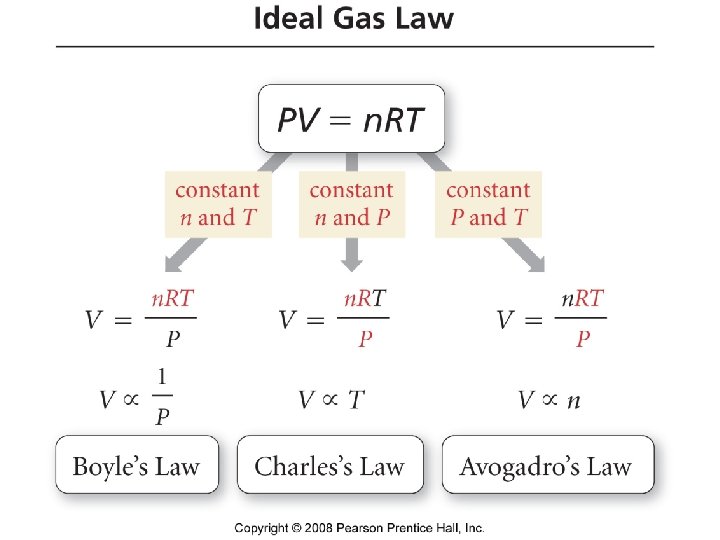
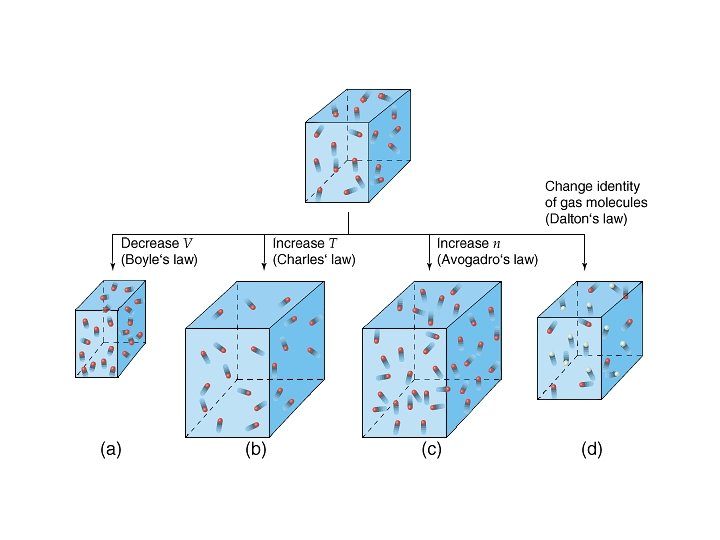
The Gas Laws · Boyles Law P 1/V • Charles Law V T • Avogadros Law V n • Combined gas law P n. T/V

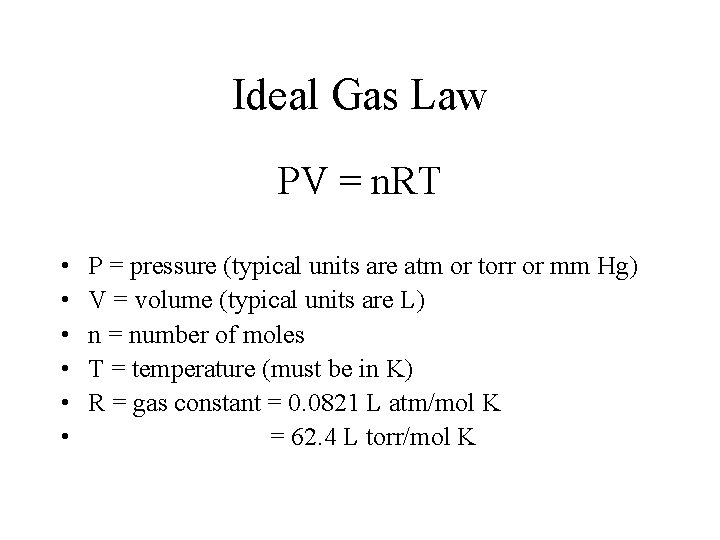
Ideal Gas Law PV = n. RT • • • P = pressure (typical units are atm or torr or mm Hg) V = volume (typical units are L) n = number of moles T = temperature (must be in K) R = gas constant = 0. 0821 L atm/mol K = 62. 4 L torr/mol K

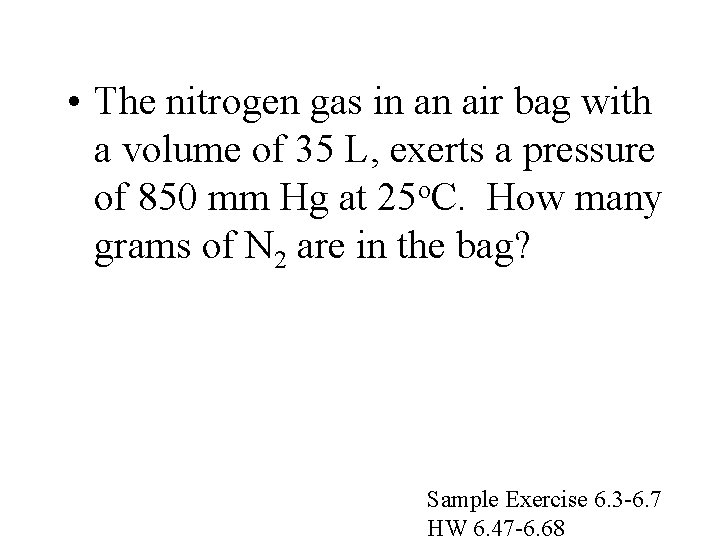
• The nitrogen gas in an air bag with a volume of 35 L, exerts a pressure of 850 mm Hg at 25 o. C. How many grams of N 2 are in the bag? Sample Exercise 6. 3 -6. 7 HW 6. 47 -6. 68

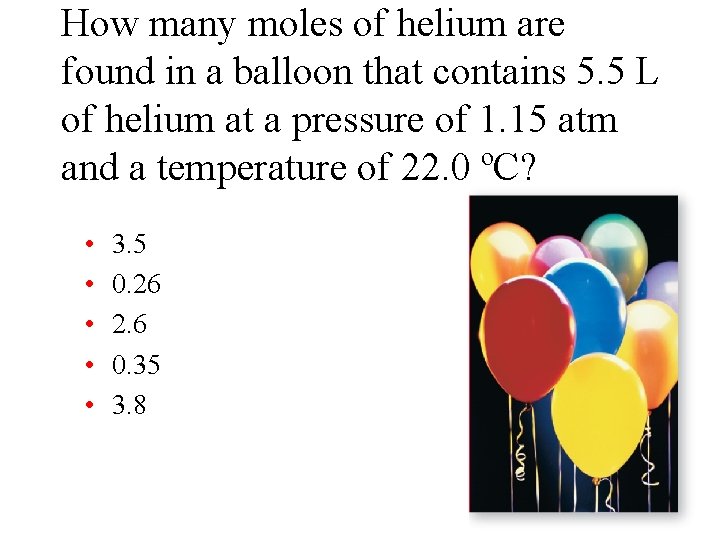
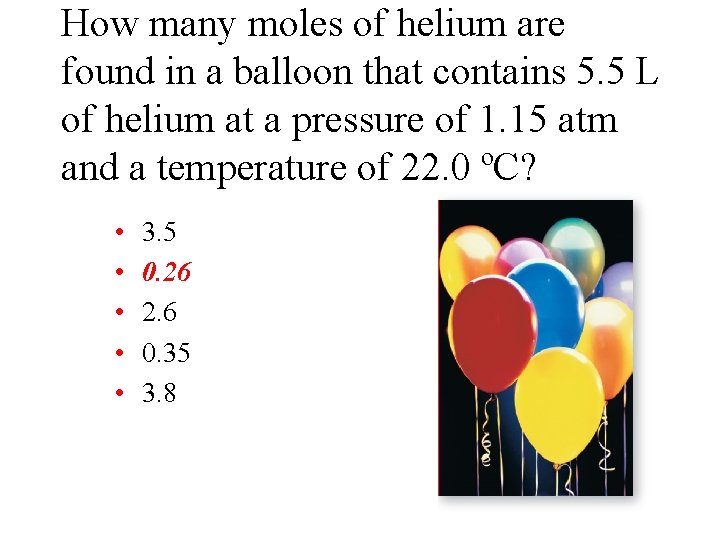
How many moles of helium are found in a balloon that contains 5. 5 L of helium at a pressure of 1. 15 atm and a temperature of 22. 0 ºC? • • • 3. 5 0. 26 2. 6 0. 35 3. 8

How many moles of helium are found in a balloon that contains 5. 5 L of helium at a pressure of 1. 15 atm and a temperature of 22. 0 ºC? • • • 3. 5 0. 26 2. 6 0. 35 3. 8

Standard Temperature and Pressure (STP) 1 atm pressure and 00 C Calculate the density of Argon gas at STP Sample exercise 6. 10, 6. 11 HW 6. 69 -6. 94

· Helium-filled balloons are used to carry scientific instruments high into the atmosphere. Suppose that a balloon is launched when the temperature is 22. 5 o. C and the barometric pressure is 754 mm Hg. If the balloon’s volume is 4. 19 x 103 L, what will it be at a height of 20 miles, where the pressure is 70. 0 mm Hg and the temperature is 33. 0 o. C?
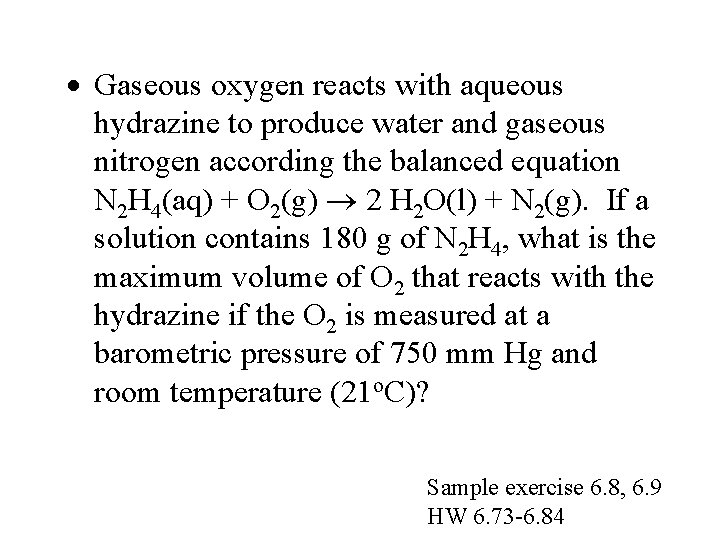
· Gaseous oxygen reacts with aqueous hydrazine to produce water and gaseous nitrogen according the balanced equation N 2 H 4(aq) + O 2(g) 2 H 2 O(l) + N 2(g). If a solution contains 180 g of N 2 H 4, what is the maximum volume of O 2 that reacts with the hydrazine if the O 2 is measured at a barometric pressure of 750 mm Hg and room temperature (21 o. C)? Sample exercise 6. 8, 6. 9 HW 6. 73 -6. 84

· Gaseous ammonia is synthesized from nitrogen and hydrogen by the reaction N 2(g) + 3 H 2(g) 2 NH 3(g) with an iron catalyst at 500 o. C. If you take 355 L of H 2 gas at 25 o. C and 542 mm. Hg and combine it with excess N 2 what volume of NH 3 gas will be collected under the same conditions?

• A mixture of CO(g) and O 2(g) in a 2. 89 L container at 907 K has a total pressure of 2. 75 atm. After time, the pressure falls to 2. 24 atm due to CO 2 formation. How many grams of CO 2 are formed? HW 6. 105 -6. 106, 6. 109 -6. 111

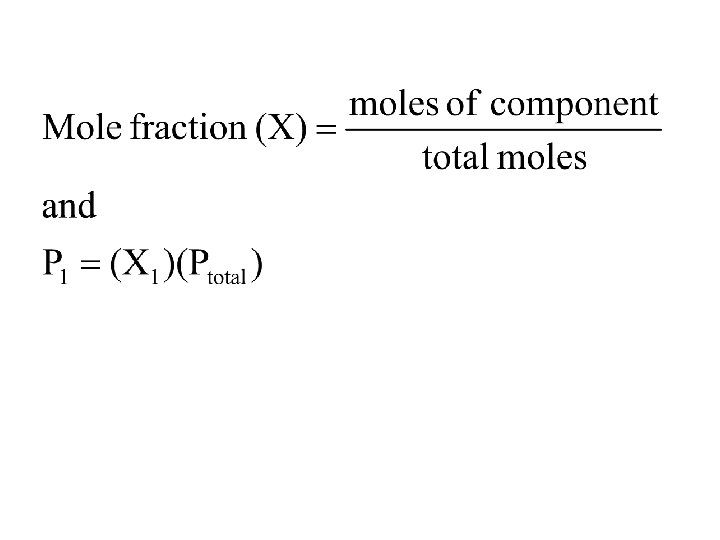
Daltons Law of Partial Pressures • Ptotal = P 1 + P 2 + P 3 +. . . + Pn


Halothane has the formula C 2 HBr. Cl. F 3. It is a nonflammable, non-explosive, and non-irritating gas that is a commonly used inhalation anesthetic. Suppose you mix 15. 0 g of haloethane vapor with 23. 5 g of oxygen gas, if the total pressure of the mixture is 855 mm. Hg, what is the mole fraction of each gas? What is the partial pressure of each gas? Sample exercise 6. 12, 6. 13 HW 6. 99 -6. 102, 6. 107 -6. 108

• A mixture of Ar and N 2 gases has a density of 1. 413 g/L at STP. What is the mole fraction of each gas?

Vapor Pressure • The partial pressure of a gas in equilibrium with liquid.

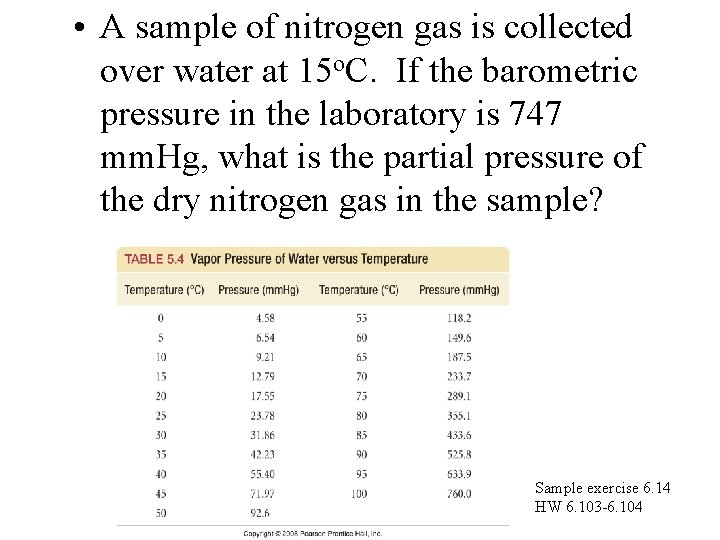
• A sample of nitrogen gas is collected over water at 15 o. C. If the barometric pressure in the laboratory is 747 mm. Hg, what is the partial pressure of the dry nitrogen gas in the sample? Sample exercise 6. 14 HW 6. 103 -6. 104

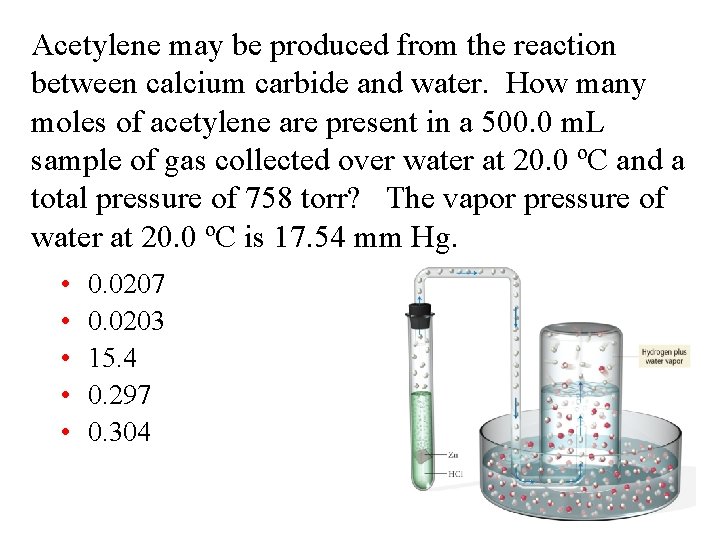
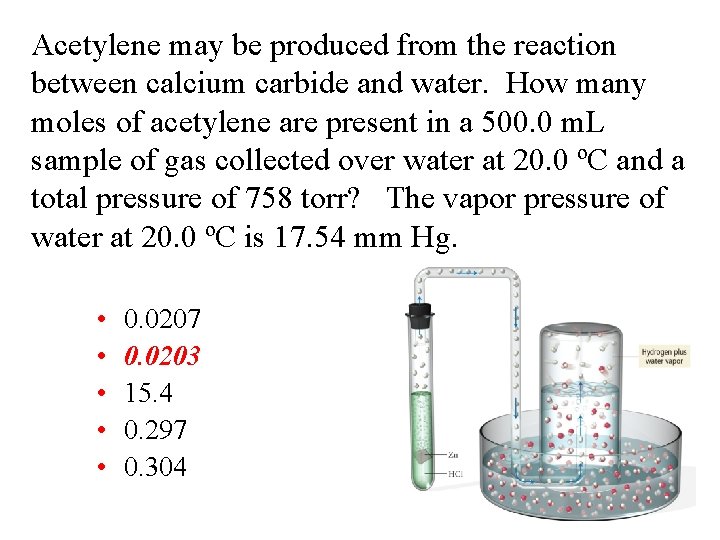
Acetylene may be produced from the reaction between calcium carbide and water. How many moles of acetylene are present in a 500. 0 m. L sample of gas collected over water at 20. 0 ºC and a total pressure of 758 torr? The vapor pressure of water at 20. 0 ºC is 17. 54 mm Hg. • • • 0. 0207 0. 0203 15. 4 0. 297 0. 304

Acetylene may be produced from the reaction between calcium carbide and water. How many moles of acetylene are present in a 500. 0 m. L sample of gas collected over water at 20. 0 ºC and a total pressure of 758 torr? The vapor pressure of water at 20. 0 ºC is 17. 54 mm Hg. • • • 0. 0207 0. 0203 15. 4 0. 297 0. 304

• Dichlorine oxide is a powerful oxidizing agent that is used to bleach wood pulp and to treat municipal water supplies. It is made by the reaction SO 2(g) + 2 Cl 2(g) SOCl 2(g) + Cl 2 O(g). If you put SO 2 in a flask so that its pressure is 125 mm. Hg at 22 o. C, and if you add Cl 2 gas to this same flask, what should the Cl 2 partial pressure be in order to have the correct stoichiometric ratio of SO 2 to Cl 2?

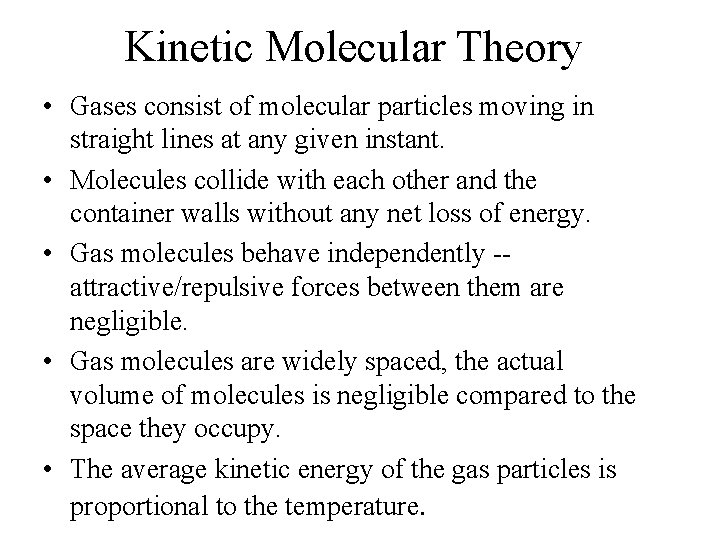
Kinetic Molecular Theory • Gases consist of molecular particles moving in straight lines at any given instant.

Kinetic Molecular Theory • Molecules collide with each other and the container walls without any net loss of energy.

Kinetic Molecular Theory • Gas molecules behave independently -attractive/repulsive forces between them are negligible.

Kinetic Molecular Theory • Gas molecules are widely spaced, the actual volume of molecules is negligible compared to the space they occupy.

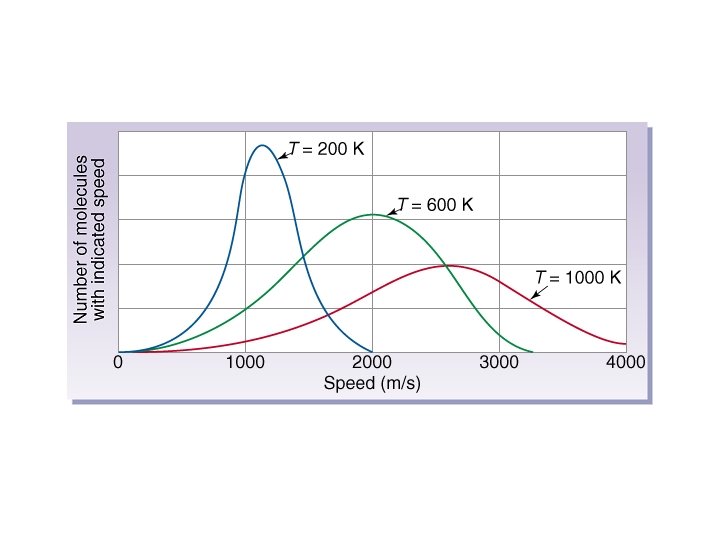
Kinetic Molecular Theory • The average kinetic energy of the gas particles is proportional to the temperature.
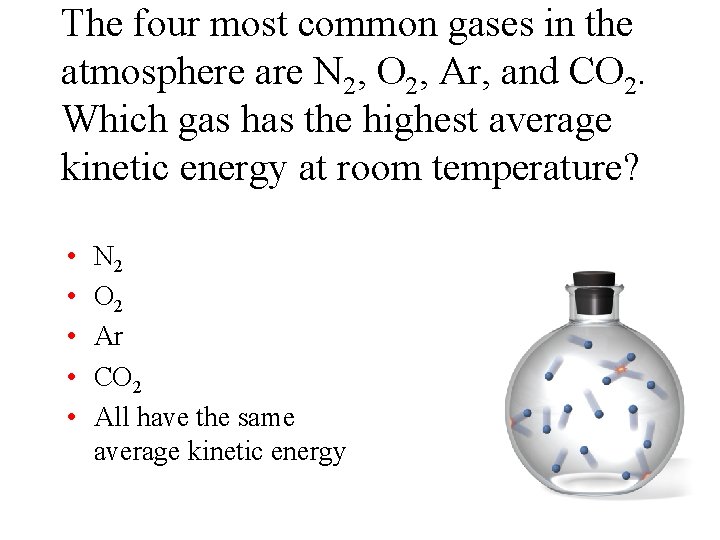
The four most common gases in the atmosphere are N 2, O 2, Ar, and CO 2. Which gas has the highest average kinetic energy at room temperature? • • • N 2 O 2 Ar CO 2 All have the same average kinetic energy

The four most common gases in the atmosphere are N 2, O 2, Ar, and CO 2. Which gas has the highest average kinetic energy at room temperature? • • • N 2 O 2 Ar CO 2 All have the same average kinetic energy



Kinetic Molecular Theory • Gases consist of molecular particles moving in straight lines at any given instant. • Molecules collide with each other and the container walls without any net loss of energy. • Gas molecules behave independently -attractive/repulsive forces between them are negligible. • Gas molecules are widely spaced, the actual volume of molecules is negligible compared to the space they occupy. • The average kinetic energy of the gas particles is proportional to the temperature.

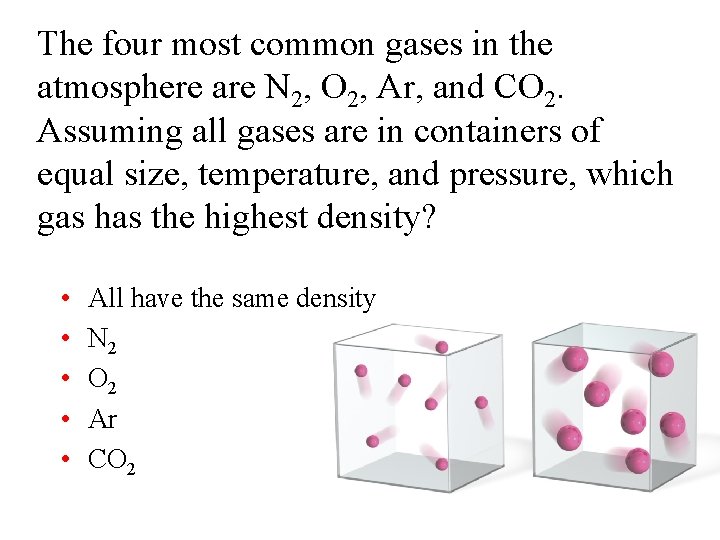
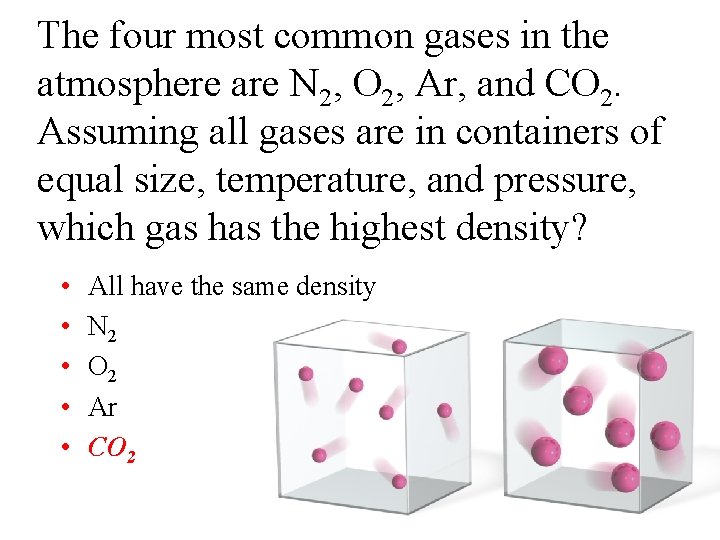
The four most common gases in the atmosphere are N 2, O 2, Ar, and CO 2. Assuming all gases are in containers of equal size, temperature, and pressure, which gas has the highest density? • • • All have the same density N 2 O 2 Ar CO 2

The four most common gases in the atmosphere are N 2, O 2, Ar, and CO 2. Assuming all gases are in containers of equal size, temperature, and pressure, which gas has the highest density? • • • All have the same density N 2 O 2 Ar CO 2

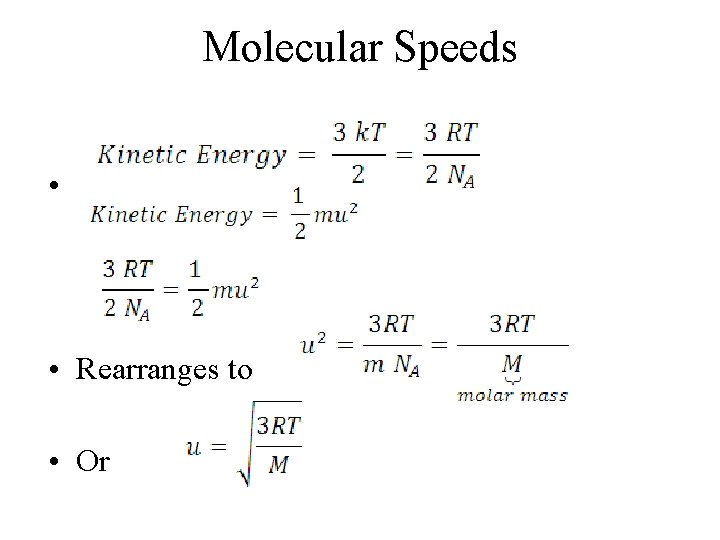
Molecular Speeds • • Rearranges to • Or
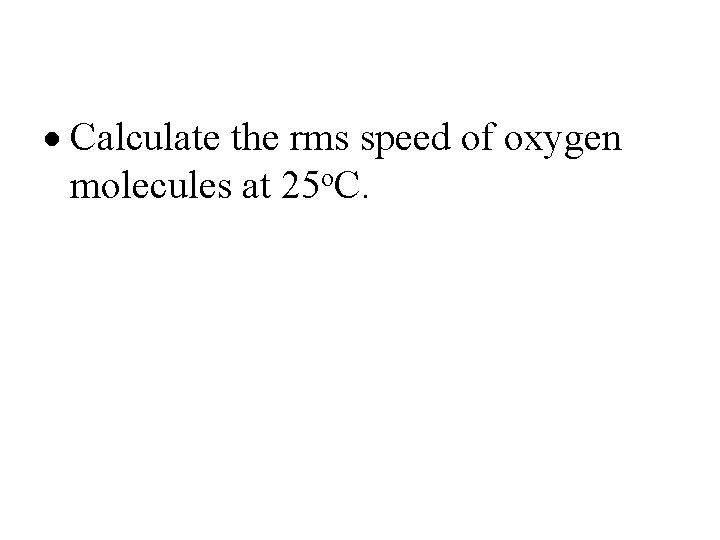
· Calculate the rms speed of oxygen molecules at 25 o. C.



· A sample of pure methane, CH 4, is found to effuse through a porous barrier in 1. 50 min. Under the same conditions, an equal number of molecules of an unknown gas effuse through the barrier in 4. 73 min. What is the molar mass of the unknown gas? Sample exercise 6. 15 -6. 16 HW 6. 121 -6. 136

• A 3. 0 L bulb containing He at 145 mm Hg is connected by a valve to a 2. 0 L bulb containing Ar at 355 mm. Hg. Calculate the partial pressure of each gas and the total pressure after the valve between the flasks is opened.






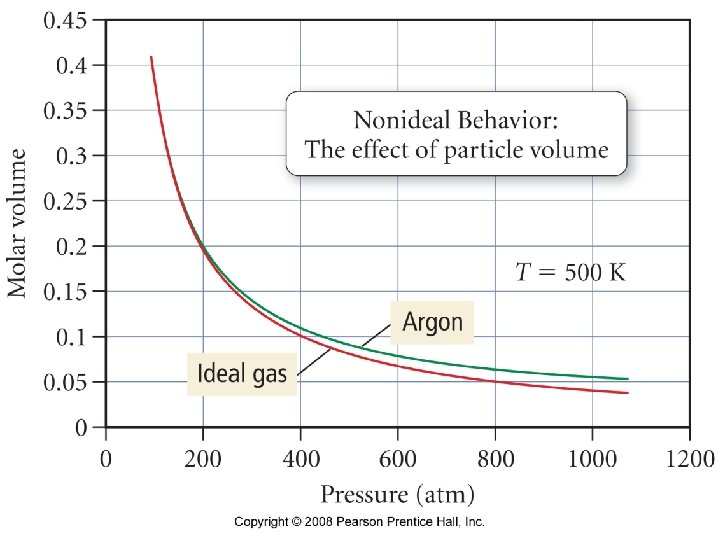
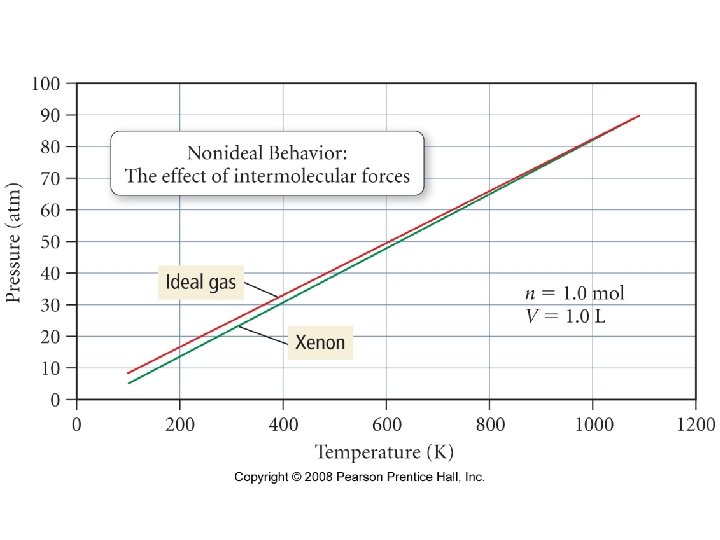
Under what conditions would Cl 2 gas be the least ideal? • High pressure and low temperature • High pressure and high temperature • Low pressure and low temperature

Under what conditions would Cl 2 gas be the least ideal? • High pressure and low temperature • High pressure and high temperature • Low pressure and low temperature

• Sample exercise 6. 18 is good!

