The Gas Laws Measuring Pressure Barometer Manometer Units

The Gas Laws

Measuring Pressure • Barometer • Manometer • Units: atmosphere, kilopascal, millimeter of mercury, Torr, Pound per square inches.

Pressure conversions • 1 atm=760 mm. Hg=101. 3 kpa=14. 7 psi
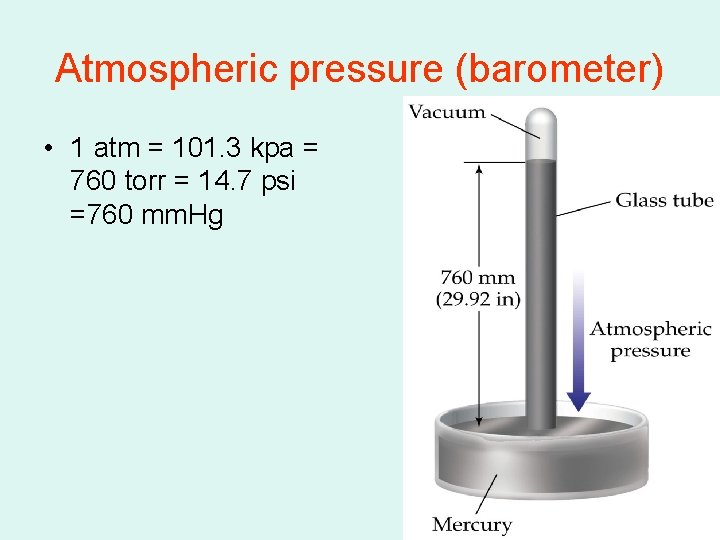
Atmospheric pressure (barometer) • 1 atm = 101. 3 kpa = 760 torr = 14. 7 psi =760 mm. Hg
Kinetic Molecular Theory • • Gases are fluids Gases have low density Gases are highly compressible Gases completely fill a container and exert pressure equally in all direction • Gases move faster in higher temperature
Atmosphere is a sea of gases • Atmosphere
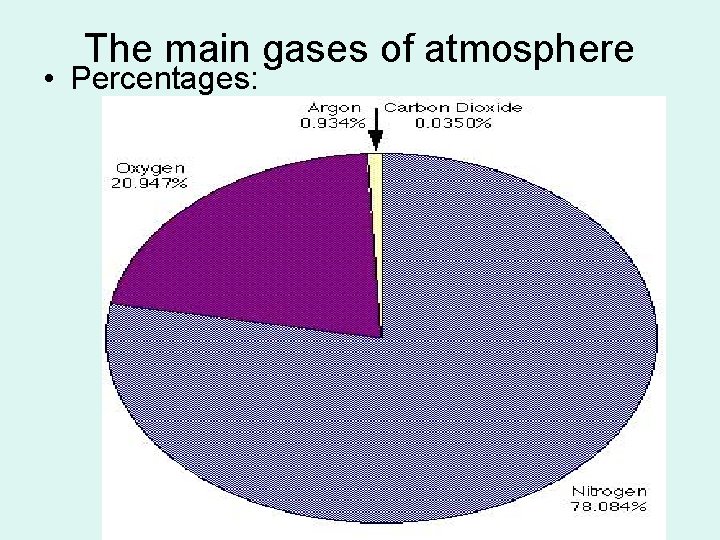
The main gases of atmosphere • Percentages:

Atmospheric pressure • Pressure= force on a surface divided by the area of that surface. Its unit in SI Pascal. • Pascal = 1 Newton of force meter squared
Standard temperature and pressure • Standard temperature and pressure STP • STP = 0°C and 1 atm • At sea level the atmospheric pressure is equal to 1 atm. • Atmospheric pressure is lower in a higher altitude and higher in the valley

Pressure conversion • Convert 22 atm into Psi • 22 atm X 14. 7 psi = 323. 4 Psi 1 atm Convert 73 k. Pa into torr 73 k. Pa X 760 torr = 547. 7 torr 101, 3 k. Pa
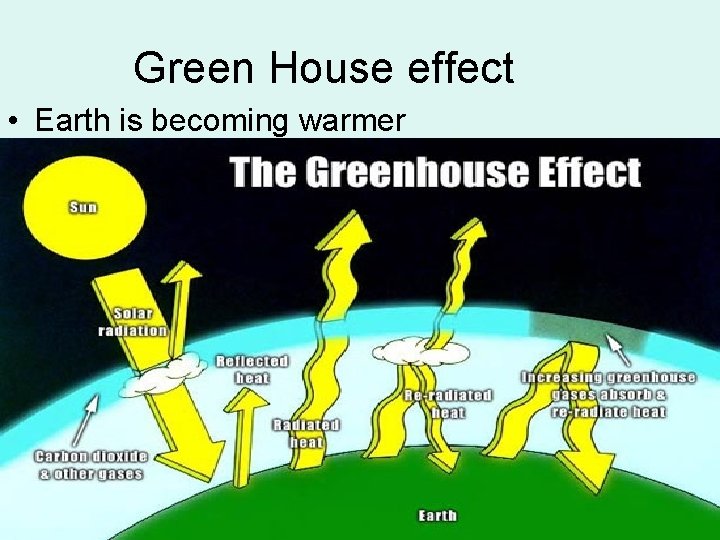
Green House effect • Earth is becoming warmer

Ozone depletion and free radicals • Free radicals such as Chlorine atom
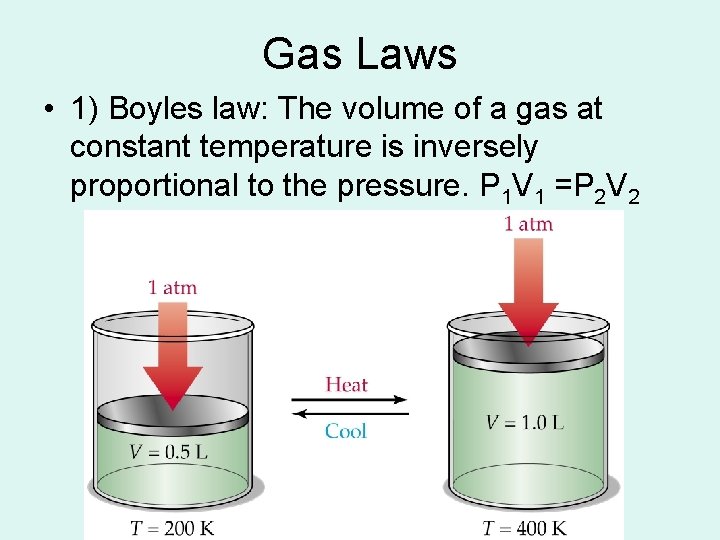
Gas Laws • 1) Boyles law: The volume of a gas at constant temperature is inversely proportional to the pressure. P 1 V 1 =P 2 V 2

Problem • A sample of a gas has a volume of 100 m. L when its pressure is 0. 947 atm. What will the volume of the gas be at a pressure of 0. 987 atm, if the temperature remains constant? P 1 V 1 =P 2 V 2 • 95. 9 m. L
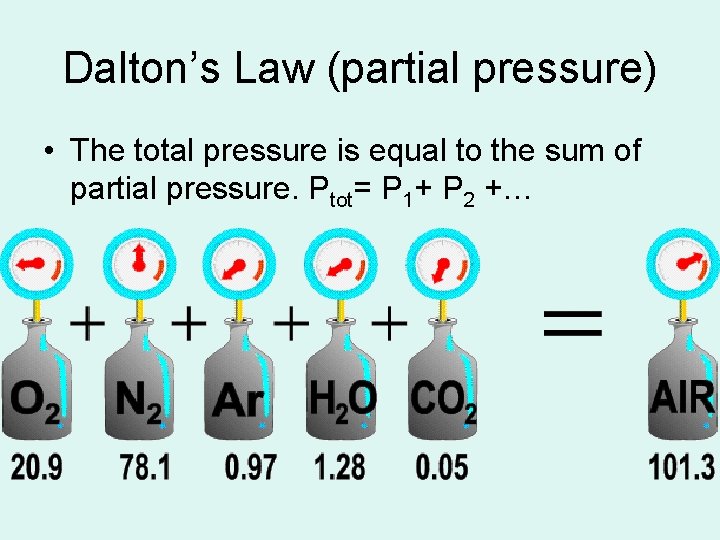
Dalton’s Law (partial pressure) • The total pressure is equal to the sum of partial pressure. Ptot= P 1+ P 2 +…

Problem • At a certain temperature, the vapor pressure of water is 20 mm. Hg. A gas is collected over water at the same temperature at a total pressure of 720 mm Hg. The partial pressure of the gas in mm. Hg is. . • 700 mm. Hg or torr
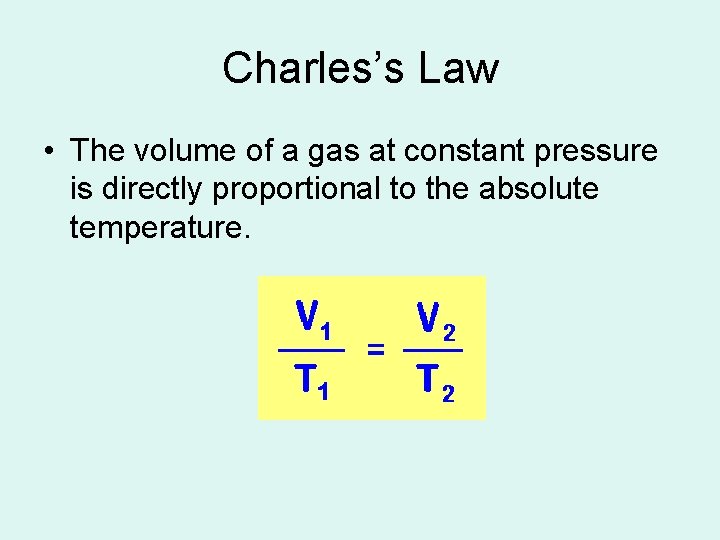
Charles’s Law • The volume of a gas at constant pressure is directly proportional to the absolute temperature.

Charles’s Law • Hot air balloon

Problem • A gas has a volume of 18 centimeter cube at 25 degree Celsius. If the temperature is increased to 35 C at constant pressure, the new volume will be. . • (Hint) T→ Kelvin = 18. 6 cm 3

Avogadro’s Law • Gases with equal volumes under the same conditions have an equal number of molecules. • I mole of any gas at STP occupies 22. 4 liter of volume. • 22. 4 liter of any gas at STP has the same number of molecules or atoms
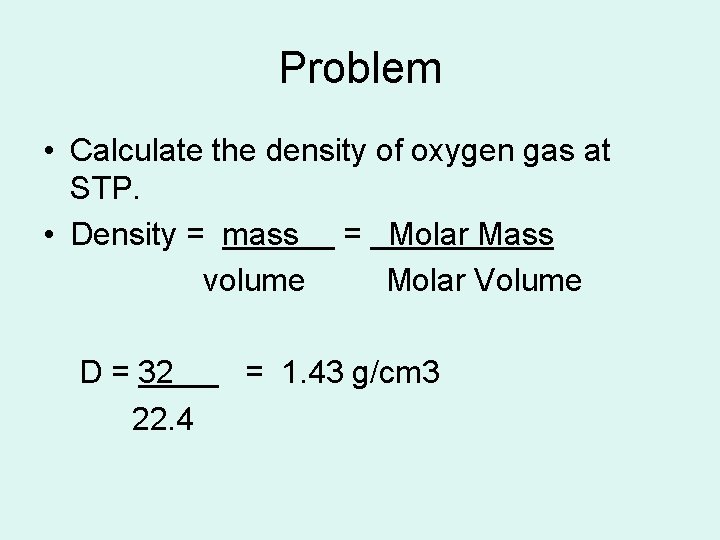
Problem • Calculate the density of oxygen gas at STP. • Density = mass = Molar Mass volume Molar Volume D = 32 22. 4 = 1. 43 g/cm 3
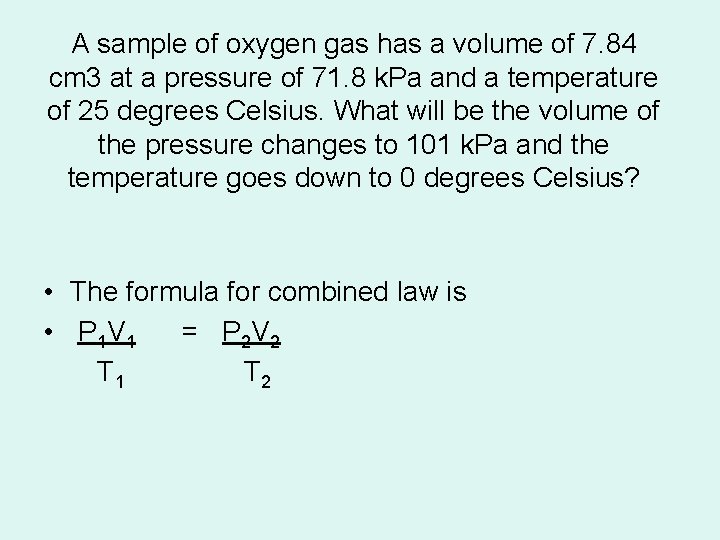
A sample of oxygen gas has a volume of 7. 84 cm 3 at a pressure of 71. 8 k. Pa and a temperature of 25 degrees Celsius. What will be the volume of the pressure changes to 101 k. Pa and the temperature goes down to 0 degrees Celsius? • The formula for combined law is • P 1 V 1 = P 2 V 2 T 1 T 2
- Slides: 22