The Gas Laws 1 Use the kineticmolecular theory






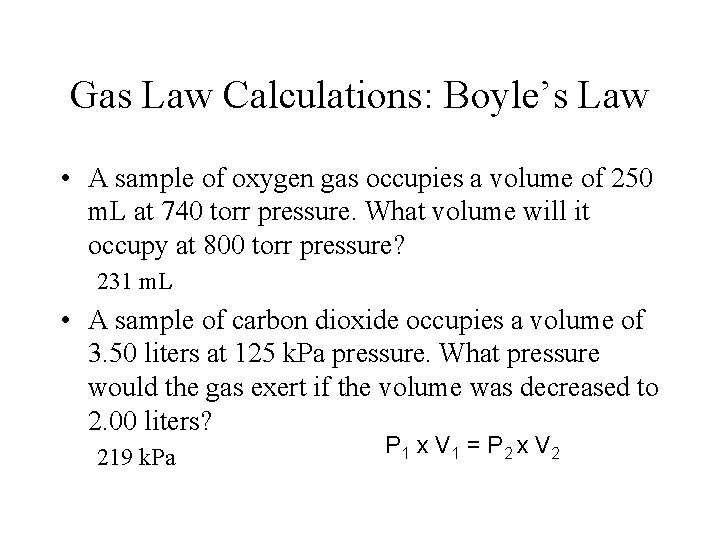







- Slides: 42

The Gas Laws 1. Use the kinetic-molecular theory to explain the behavior of gases 2. State, apply, & calculate the 3 following gas laws: a. Boyle’s Law b. Charles’s Law c. Gay-Lussac’s Law

The Combined Gas Law & Avogadro’s Principle 1. State, apply, & calculate the combined gas law 2. Relate number of particles and volumes using Avogadro’s Principle

The Ideal Gas Law 1. State, apply, & calculate the ideal gas law 2. State, apply, & calculate Dalton’s Law of Partial Pressure 3. State, apply, & calculate Graham’s Law of Effusion http: //www. chemistry. ohio-state. edu/betha/neal. Gas. Law/fr 2. 1. html

BR: Visit the following site! http: //www. mhhe. com/physsci/chemistry/animations/chang_7 e_esp/gam 2 s 2_6. swf

Science Connection • From barbecuing on a gas grill to taking a ride in a hot air balloon, many activities involve gases. • It is important to be able to predict what effect changes in pressure, temperature, volume, or amount will have on the properties and behavior of a gas. Gases expand, diffuse, exert pressure, and can be compressed because they are in a low density state consisting of tiny, constantly-moving particles.

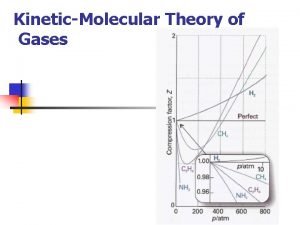
Gases Chs. 12, 13 STP is standard temperature and pressure • Kinetic-molecular theory – Explains the properties of gases – 5 assumptions/Characteristics of gases • • Gases are fluids Gases have low density Gases are highly compressible Gases completely fill a container and exert pressure equally in all directions • The temp of a gas determines the average KE of its particles – Recall from PSci KE = ½mv 2

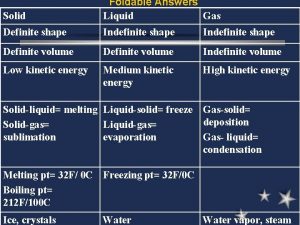
The Kinetic-Molecular Theory Section 12 -1 • Kinetic-molecular theory explains the different properties of solids, liquids, and gases. • Atomic composition affects chemical properties. • Atomic composition also affects physical properties. • The kinetic-molecular theory describes the behavior of matter in terms of particles in motion.

The Kinetic-Molecular Theory (cont. ) Section 12 -1 • Gases consist of small particles separated by empty space. • Gas particles are too far apart to experience significant attractive or repulsive forces.

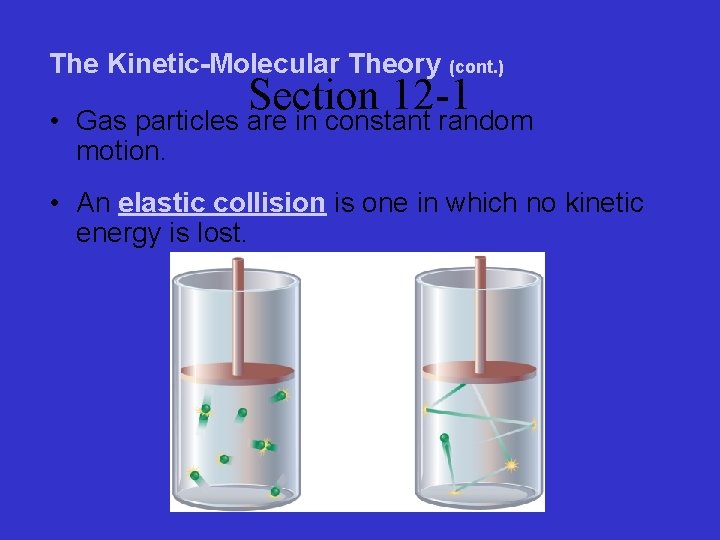
The Kinetic-Molecular Theory (cont. ) • Section 12 -1 Gas particles are in constant random motion. • An elastic collision is one in which no kinetic energy is lost.

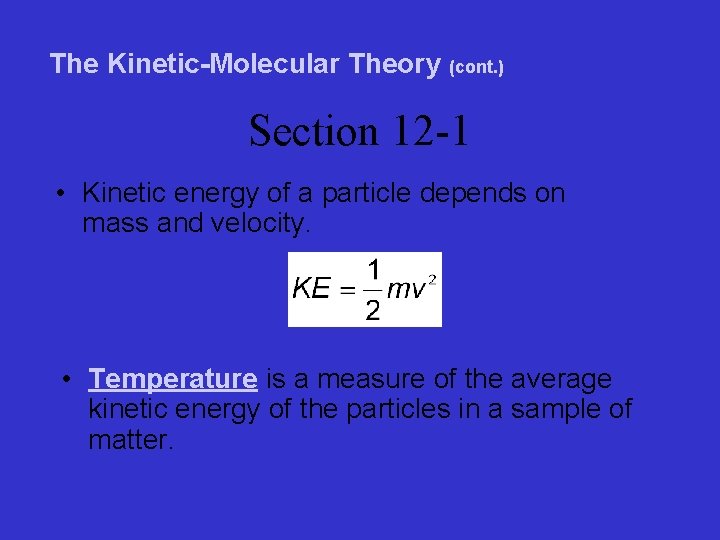
The Kinetic-Molecular Theory (cont. ) Section 12 -1 • Kinetic energy of a particle depends on mass and velocity. • Temperature is a measure of the average kinetic energy of the particles in a sample of matter.

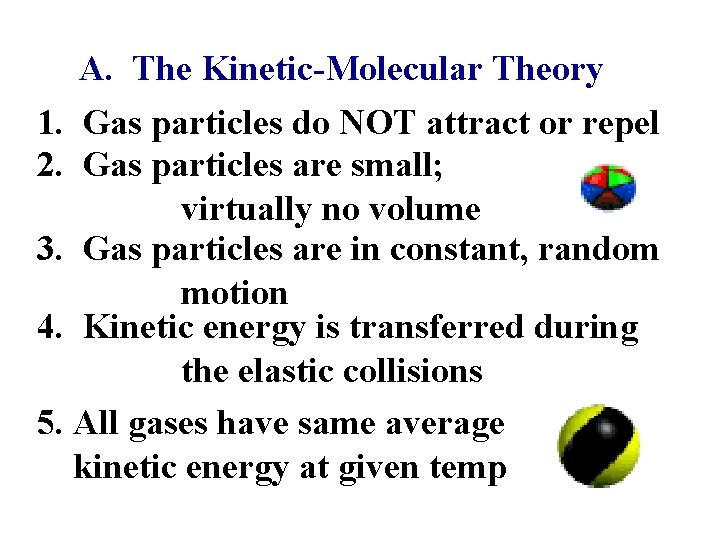
A. The Kinetic-Molecular Theory 1. Gas particles do NOT attract or repel 2. Gas particles are small; virtually no volume 3. Gas particles are in constant, random motion 4. Kinetic energy is transferred during the elastic collisions 5. All gases have same average kinetic energy at given temp

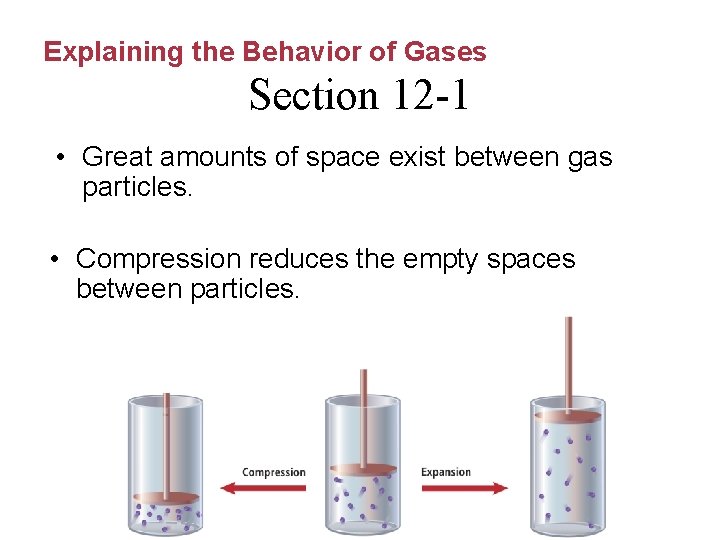
Explaining the Behavior of Gases Section 12 -1 • Great amounts of space exist between gas particles. • Compression reduces the empty spaces between particles.

Explaining the Behavior of Gases (cont. ) Section 12 -1 • Gases easily flow past each other because there are no significant forces of attraction. • Diffusion is the movement of one material through another. • Effusion is a gas escaping through a tiny opening.

B. The Nature of Gases 1. Actual gases do not always obey the kinetic-molecular theory 2. The KMT is based on 4 factors: a. Temperature (measured in Kelvin) [o. C + 273 = K] b. Pressure (measured in atm, k. Pa, etc. ) [1 atm = 101. 3 k. Pa = 760 mm. Hg or torr] c. Volume (measured in liters or m. L) d. Amount of Gas (measured in moles)

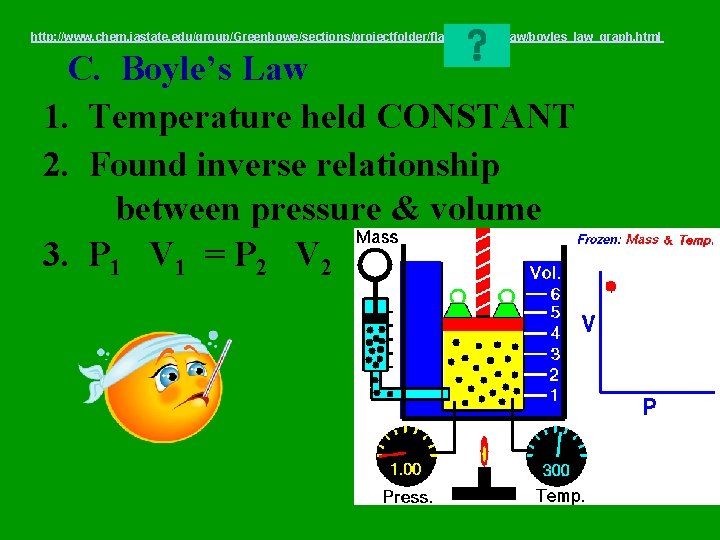
http: //www. chem. iastate. edu/group/Greenbowe/sections/projectfolder/flashfiles/gaslaw/boyles_law_graph. html C. Boyle’s Law 1. Temperature held CONSTANT 2. Found inverse relationship between pressure & volume 3. P 1 V 1 = P 2 V 2

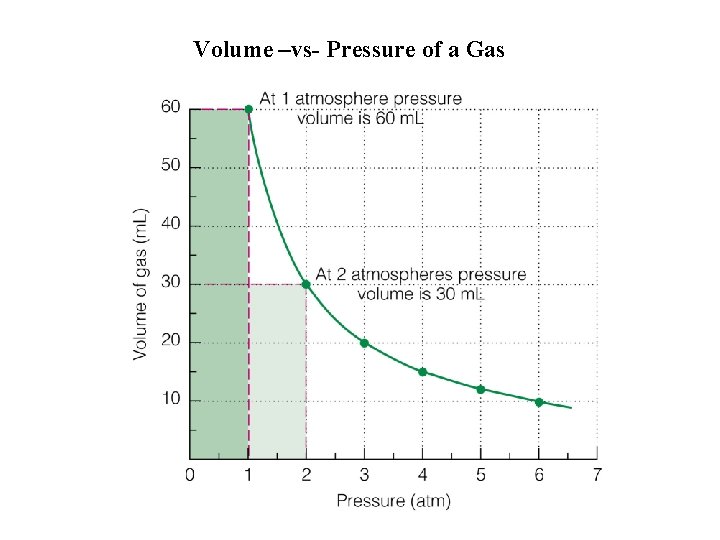
Volume –vs- Pressure of a Gas

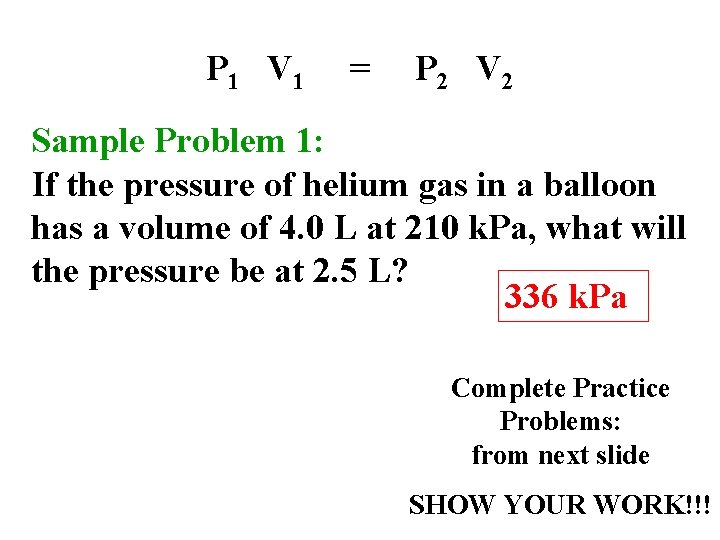
P 1 V 1 = P 2 V 2 Sample Problem 1: If the pressure of helium gas in a balloon has a volume of 4. 0 L at 210 k. Pa, what will the pressure be at 2. 5 L? 336 k. Pa Complete Practice Problems: from next slide SHOW YOUR WORK!!!
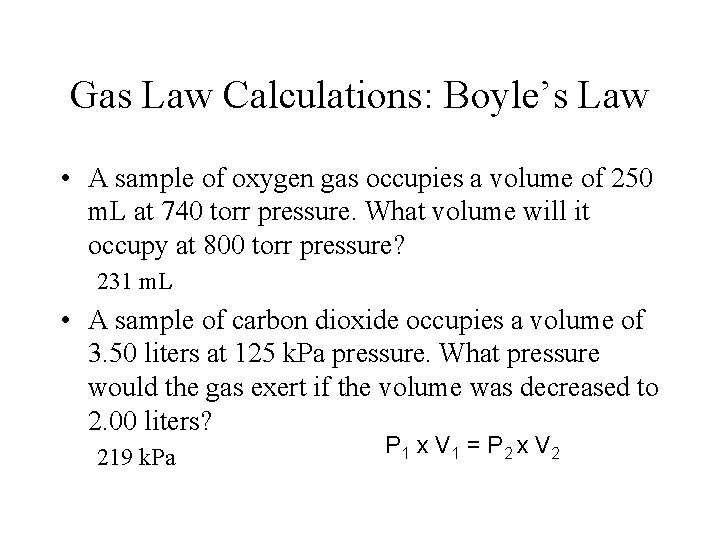
Gas Law Calculations: Boyle’s Law • A sample of oxygen gas occupies a volume of 250 m. L at 740 torr pressure. What volume will it occupy at 800 torr pressure? 231 m. L • A sample of carbon dioxide occupies a volume of 3. 50 liters at 125 k. Pa pressure. What pressure would the gas exert if the volume was decreased to 2. 00 liters? 219 k. Pa P 1 x V 1 = P 2 x V 2

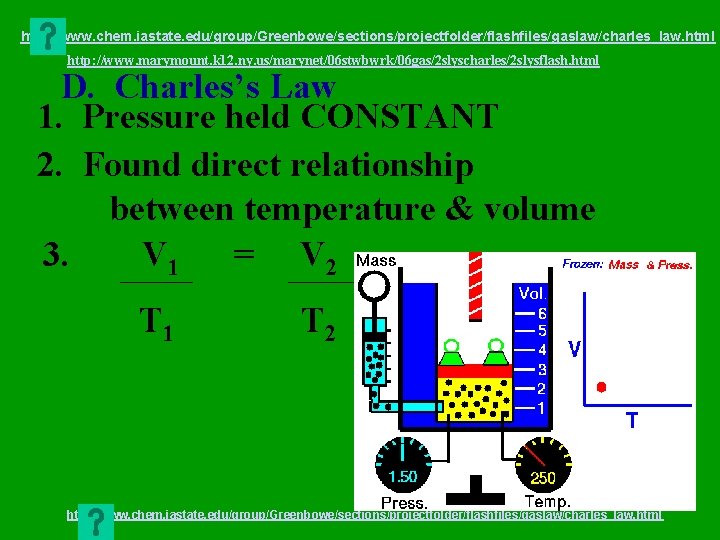
http: //www. chem. iastate. edu/group/Greenbowe/sections/projectfolder/flashfiles/gaslaw/charles_law. html http: //www. marymount. k 12. ny. us/marynet/06 stwbwrk/06 gas/2 slyscharles/2 slysflash. html D. Charles’s Law 1. Pressure held CONSTANT 2. Found direct relationship between temperature & volume 3. V 1 = V 2 T 1 T 2 http: //www. chem. iastate. edu/group/Greenbowe/sections/projectfolder/flashfiles/gaslaw/charles_law. html

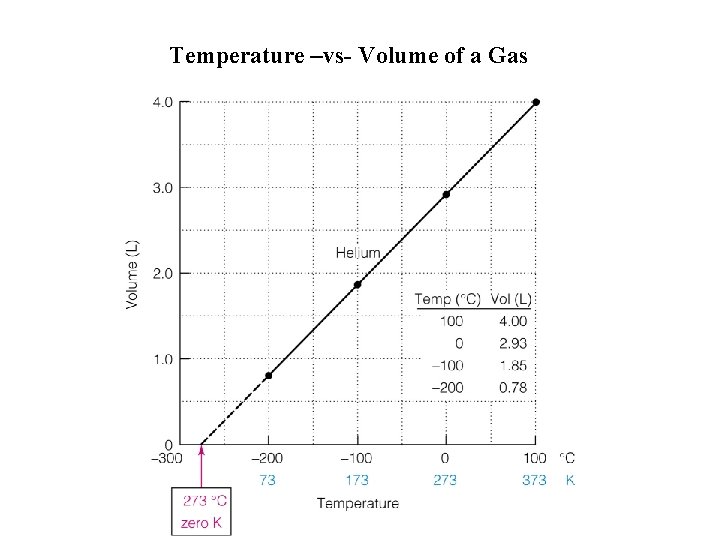
Temperature –vs- Volume of a Gas

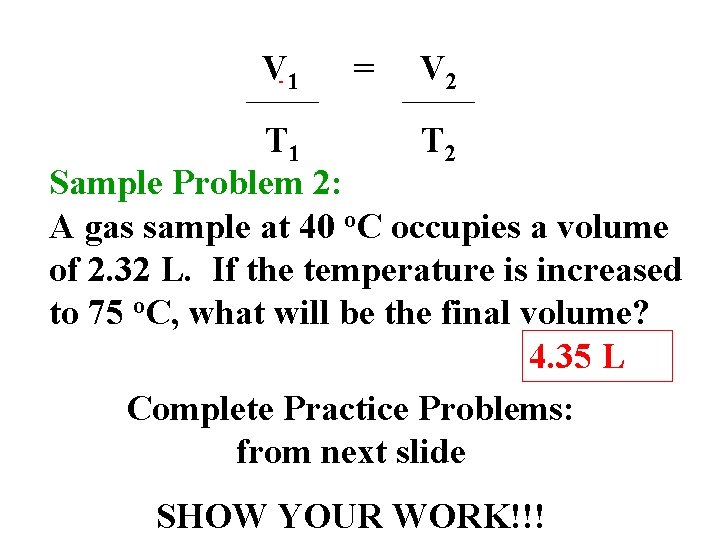
V 1 = V 2 T 1 T 2 Sample Problem 2: A gas sample at 40 o. C occupies a volume of 2. 32 L. If the temperature is increased to 75 o. C, what will be the final volume? 4. 35 L Complete Practice Problems: from next slide SHOW YOUR WORK!!!

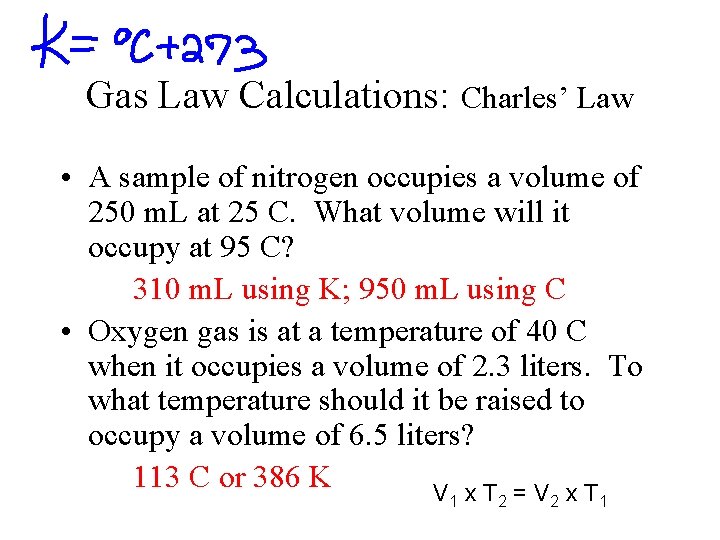
Gas Law Calculations: Charles’ Law • A sample of nitrogen occupies a volume of 250 m. L at 25 C. What volume will it occupy at 95 C? 310 m. L using K; 950 m. L using C • Oxygen gas is at a temperature of 40 C when it occupies a volume of 2. 3 liters. To what temperature should it be raised to occupy a volume of 6. 5 liters? 113 C or 386 K V x. T =V x. T 1 2 2 1

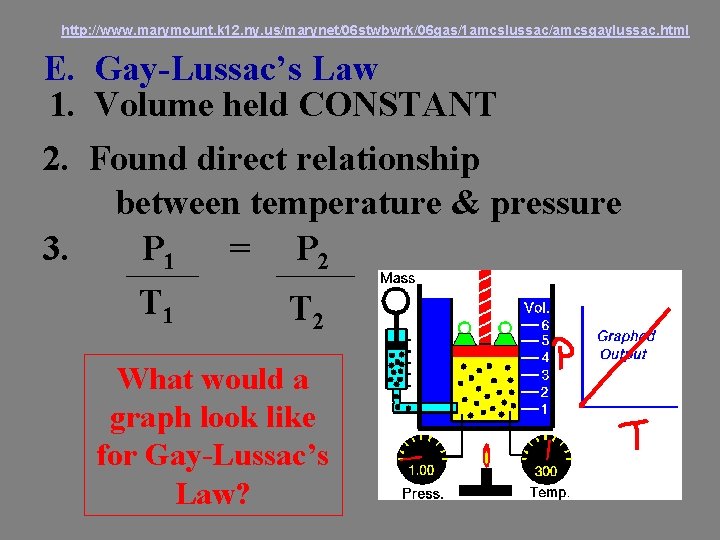
http: //www. marymount. k 12. ny. us/marynet/06 stwbwrk/06 gas/1 amcslussac/amcsgaylussac. html E. Gay-Lussac’s Law 1. Volume held CONSTANT 2. Found direct relationship between temperature & pressure 3. P 1 = P 2 T 1 T 2 What would a graph look like for Gay-Lussac’s Law?

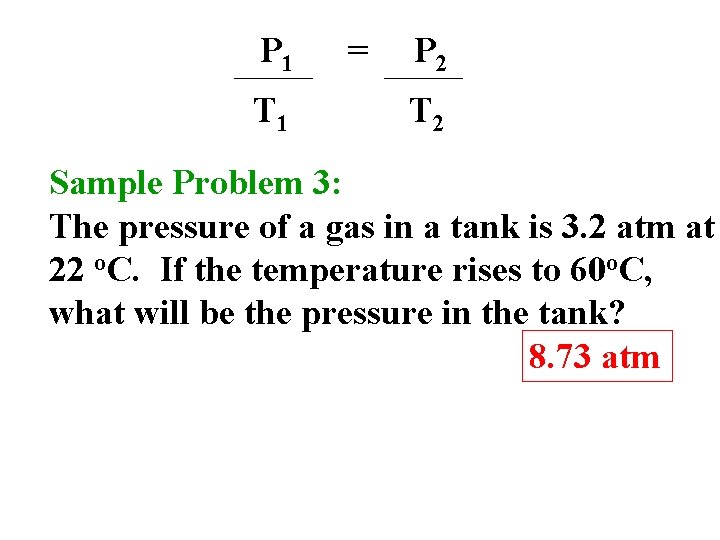
P 1 T 1 = P 2 T 2 Sample Problem 3: The pressure of a gas in a tank is 3. 2 atm at 22 o. C. If the temperature rises to 60 o. C, what will be the pressure in the tank? 8. 73 atm

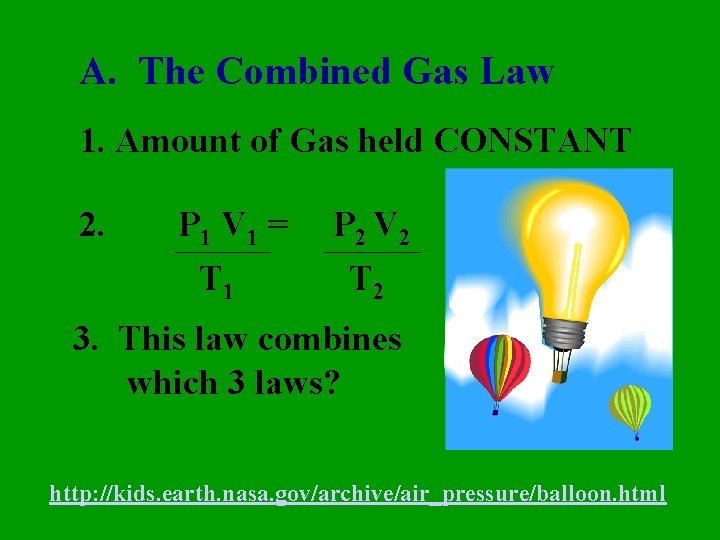
A. The Combined Gas Law 1. Amount of Gas held CONSTANT 2. P 1 V 1 = T 1 P 2 V 2 T 2 3. This law combines which 3 laws? http: //kids. earth. nasa. gov/archive/air_pressure/balloon. html

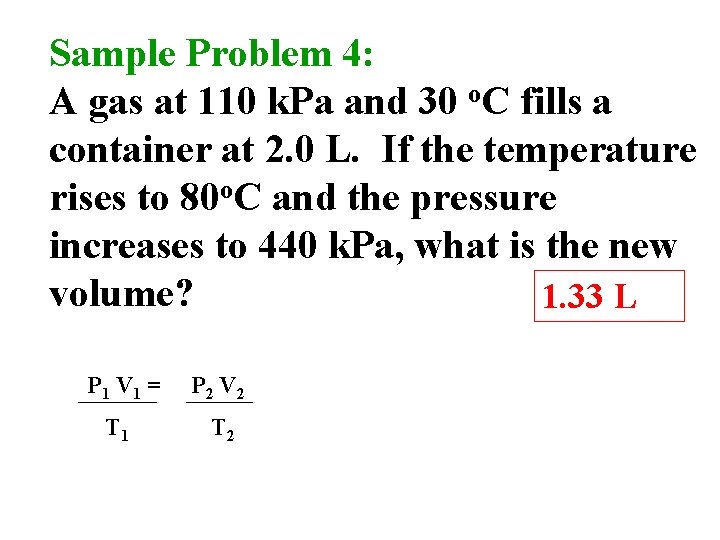
Sample Problem 4: A gas at 110 k. Pa and 30 o. C fills a container at 2. 0 L. If the temperature rises to 80 o. C and the pressure increases to 440 k. Pa, what is the new volume? 1. 33 L P 1 V 1 = P 2 V 2 T 1 T 2

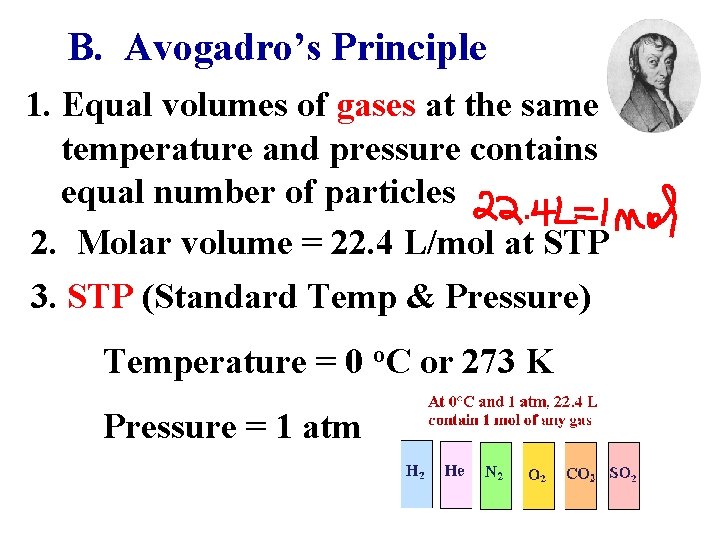
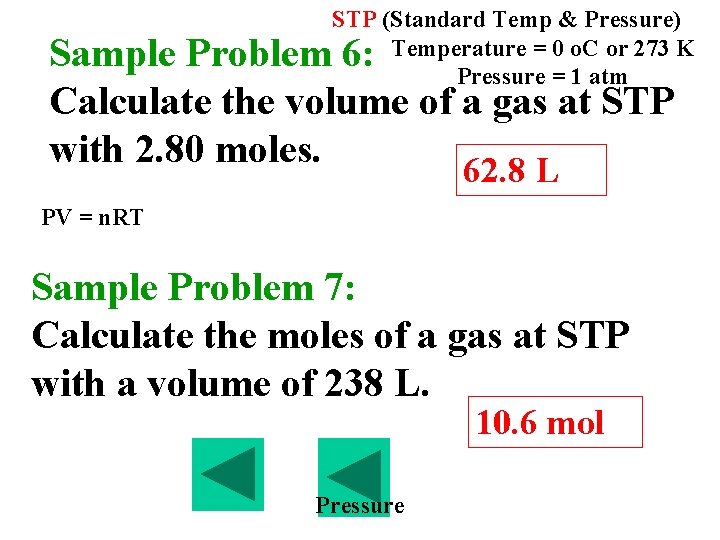
B. Avogadro’s Principle 1. Equal volumes of gases at the same temperature and pressure contains equal number of particles 2. Molar volume = 22. 4 L/mol at STP 3. STP (Standard Temp & Pressure) Temperature = 0 o. C or 273 K Pressure = 1 atm

STP (Standard Temp & Pressure) Temperature = 0 o. C or 273 K Pressure = 1 atm Sample Problem 5: Calculate the volume that 0. 881 mol of a gas at STP will occupy. 19. 7 L

Real Versus Ideal Gases Section 13 -2 • Ideal gases follow the assumptions of the kinetic-molecular theory. • Ideal gases experience: – There are no intermolecular attractive or repulsive forces between particles or with their containers. – The particles are in constant random motion. – Collisions are perfectly elastic. – No gas is truly ideal, but most behave as ideal gases at a wide range of temperatures and pressures.

Real Versus Ideal Gases (cont. ) Section 13 -2 • Real gases deviate most from ideal gases at high pressures and low temperatures. • Polar molecules have larger attractive forces between particles. • Polar gases do not behave as ideal gases. • Large nonpolar gas particles occupy more space and deviate more from ideal gases.

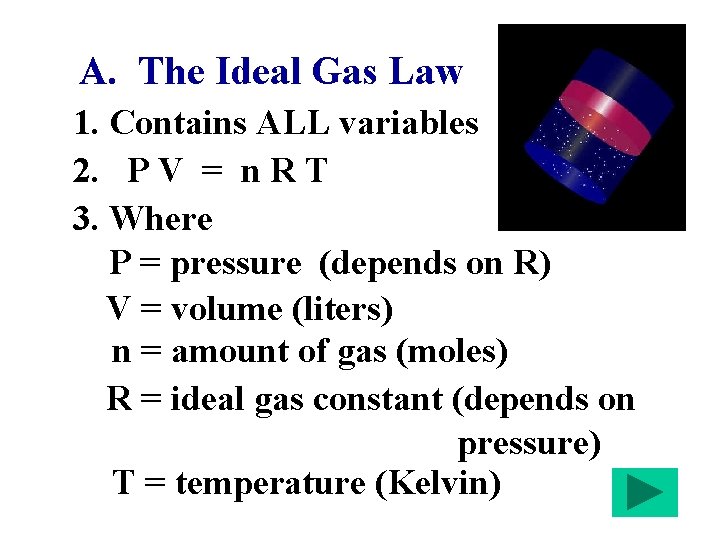
A. The Ideal Gas Law 1. Contains ALL variables 2. P V = n R T 3. Where P = pressure (depends on R) V = volume (liters) n = amount of gas (moles) R = ideal gas constant (depends on pressure) T = temperature (Kelvin)

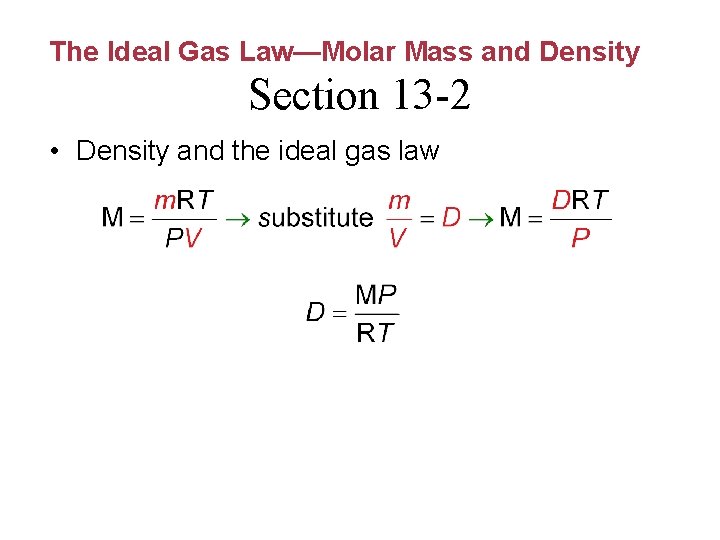
The Ideal Gas Law—Molar Mass and Density Section 13 -2 • Density and the ideal gas law

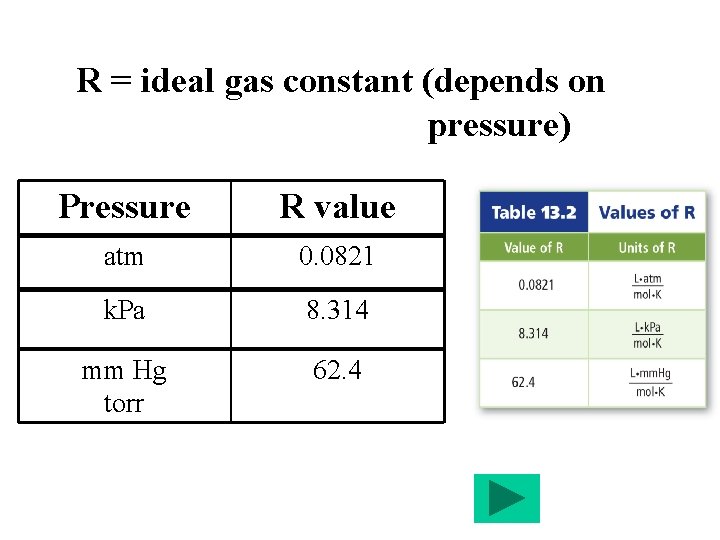
R = ideal gas constant (depends on pressure) Pressure R value atm 0. 0821 k. Pa 8. 314 mm Hg torr 62. 4


STP (Standard Temp & Pressure) Temperature = 0 o. C or 273 K Pressure = 1 atm Sample Problem 6: Calculate the volume of a gas at STP with 2. 80 moles. 62. 8 L PV = n. RT Sample Problem 7: Calculate the moles of a gas at STP with a volume of 238 L. 10. 6 mol Pressure

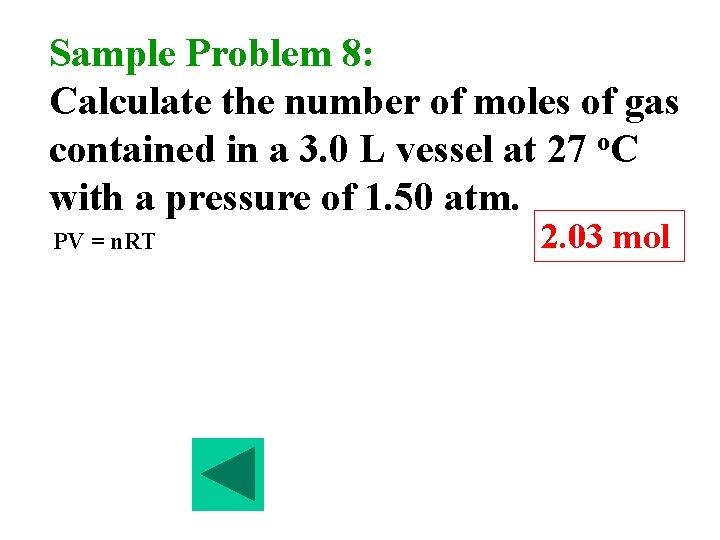
Sample Problem 8: Calculate the number of moles of gas contained in a 3. 0 L vessel at 27 o. C with a pressure of 1. 50 atm. PV = n. RT 2. 03 mol

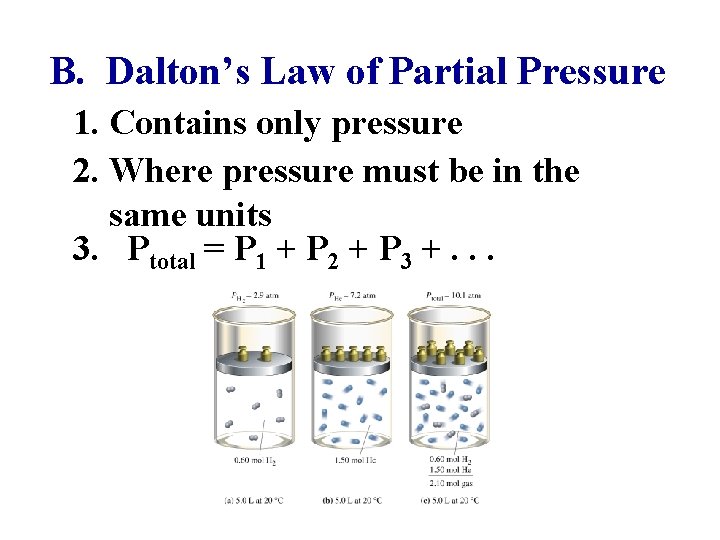
B. Dalton’s Law of Partial Pressure 1. Contains only pressure 2. Where pressure must be in the same units 3. Ptotal = P 1 + P 2 + P 3 +. . .

Ptotal = P 1 + P 2 + P 3 +. . . 4. Sample Problem 9: If the total pressure of a mixture of oxygen & nitrogen gases was 820 mm. Hg, how much pressure would nitrogen exert if oxygen had 580 mm. Hg? 240 mm. Hg

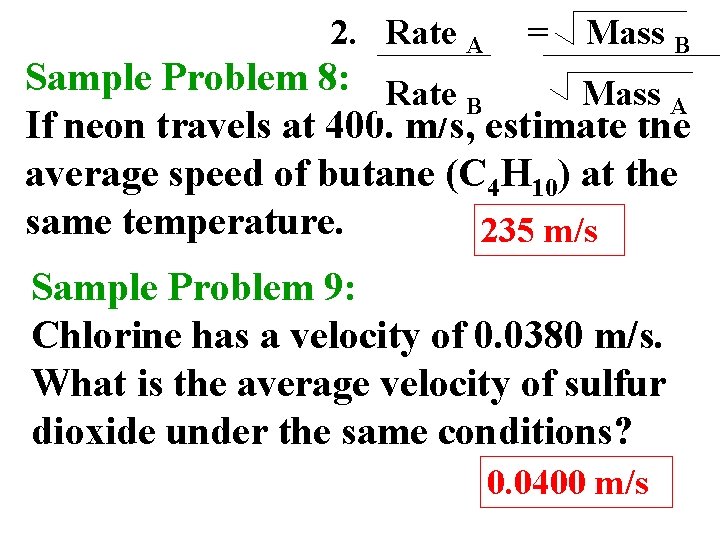
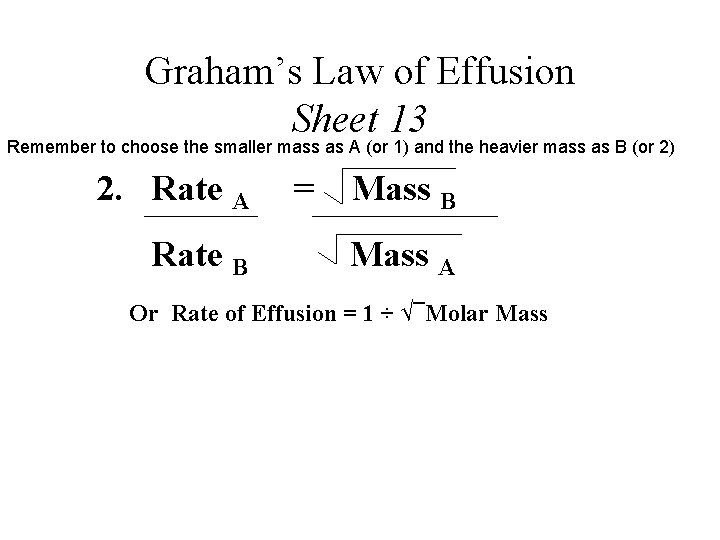
2. Rate A = Mass B Sample Problem 8: Rate Mass A B If neon travels at 400. m/s, estimate the average speed of butane (C 4 H 10) at the same temperature. 235 m/s Sample Problem 9: Chlorine has a velocity of 0. 0380 m/s. What is the average velocity of sulfur dioxide under the same conditions? 0. 0400 m/s

Graham’s Law of Effusion Sheet 13 Remember to choose the smaller mass as A (or 1) and the heavier mass as B (or 2) 2. Rate A Rate B = Mass B Mass A Or Rate of Effusion = 1 ÷ √¯Molar Mass

Gas Stoichiometry • Calculations involving only volume – Use eqn mole ratios • Coefficient = moles = volumes called vol ratios Other conversion factors 1 mol = 22. 4 L @ STP 1 atm = 101, 325 Pa 1 torr = 133. 322 Pa = 1 mm Hg Any other conversion can be made after this!!! You Try Practice Problems

What Did I Learn Today? • Kinetic theory of gases (kinetic-molecular theory) and the Kelvin scale • Heating and cooling curve interpretation and construction • The behavior of gases • Characteristics of gases • PTV and the gas laws • The combined gas law – Avogadro’s principle • Ideal gas law – Dalton’s law of partial pressure – Graham’s law of effusion
 Charles de secondat
Charles de secondat Gas laws crash course
Gas laws crash course Boyle's law indirect or direct
Boyle's law indirect or direct What are the empirical gas laws
What are the empirical gas laws Gas laws formula
Gas laws formula All the gas laws
All the gas laws Different gas laws
Different gas laws Charles law
Charles law Gas law conceptual questions
Gas law conceptual questions Example of boyle's law problem
Example of boyle's law problem Combined gas law
Combined gas law Boyle's gas law formula
Boyle's gas law formula Different gas laws
Different gas laws Combined gas laws
Combined gas laws Which gas law relates pressure and temperature
Which gas law relates pressure and temperature Boyle's law states that
Boyle's law states that Empirical gas laws
Empirical gas laws Ap chemistry gas laws
Ap chemistry gas laws Gas law graphic organizer
Gas law graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Gay lussac's law in real life
Gay lussac's law in real life Kmt gas laws
Kmt gas laws Gas law formula
Gas law formula Empirical gas law
Empirical gas law Gas variable relationships
Gas variable relationships All gas laws
All gas laws Is gas definite or indefinite
Is gas definite or indefinite Ideal gas vs perfect gas
Ideal gas vs perfect gas Imaginary gas
Imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Computational fluid dynamics
Computational fluid dynamics Reason for bhopal gas tragedy
Reason for bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Volume molare
Volume molare Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Persamaan arrhenius
Persamaan arrhenius Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Partitions of a set
Partitions of a set Vir
Vir Example of a parallel circuit
Example of a parallel circuit Idempotent law truth table
Idempotent law truth table