The Fraunhofer Institute fr Cell Therapy und Immunology



- Slides: 17

The Fraunhofer Institute für Cell Therapy und Immunology Applied Research in Leipzig Bridging the gap between the Czech Republic and Germany in the Life Science sector Sonya Faber Fraunhofer Institut für Zelltherapie und Immunologie Joint Workshop of the BMBF and the MSMT Prague, 7 th of December, 2006

Summary About the Fraunhofer Institute Opportunities in the Czech Republic Opportunities for Fraunhofer Possible Synergy

The Fraunhofer Society Joseph von Fraunhofer (1787 - 1826) Researcher Mission Applied research to support industry the service sector and the government Cooperation Partners/Clients Inventor n Industry and Service Providers n Government Research Entrepreneur Innovative research for public and commercial interests annual research budget of 1 billion euro

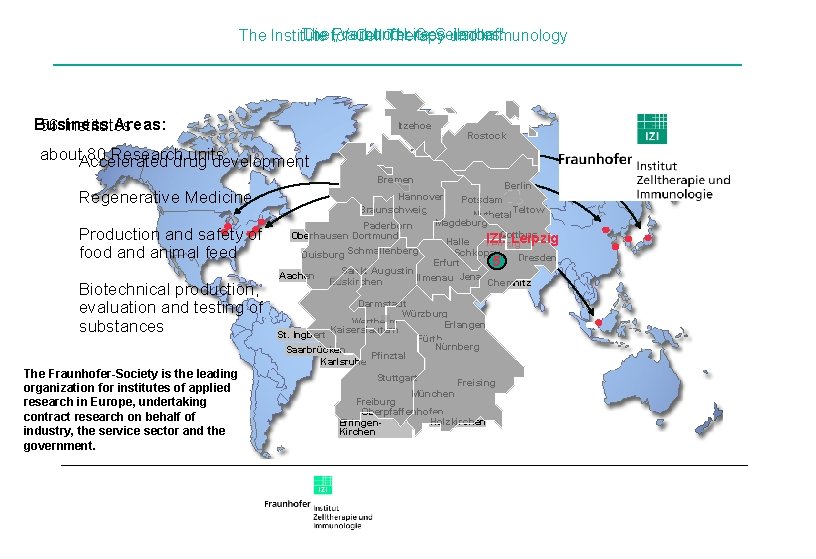
Die for Fraunhofer-Gesellschaft The „Verbund Life Sciences“ The Institute Cell Therapy und Immunology Business Areas: 56 Institutes Itzehoe Rostock about. Accelerated 80 Research units drug development Bremen Regenerative Medicine Production and safety of food animal feed Biotechnical production, evaluation and testing of substances The Fraunhofer-Society is the leading organization for institutes of applied research in Europe, undertaking contract research on behalf of industry, the service sector and the government. Berlin Hannover Potsdam Teltow Braunschweig Nuthetal Magdeburg Paderborn Cottbus Oberhausen Dortmund IZI: Leipzig Halle Leipzig Schmallenberg Schkopau Duisburg Erfurt 5 Dresden Sankt Augustin Aachen Ilmenau Jena Euskirchen Chemnitz Darmstadt Würzburg Wertheim Erlangen Kaiserslautern St. Ingbert Fürth Nürnberg Saarbrücken Pfinztal Karlsruhe Stuttgart München Freising Freiburg Oberpfaffenhofen Holzkirchen Efringen. Kirchen

The Institute for Cell Therapie and Immunology • 200 Staff Members • 1600 m 2 Laboratory area • 450 m 2 GMP area • 1600 m 2 Office area • 24 Mio. Euro • Completion: Spring 2008

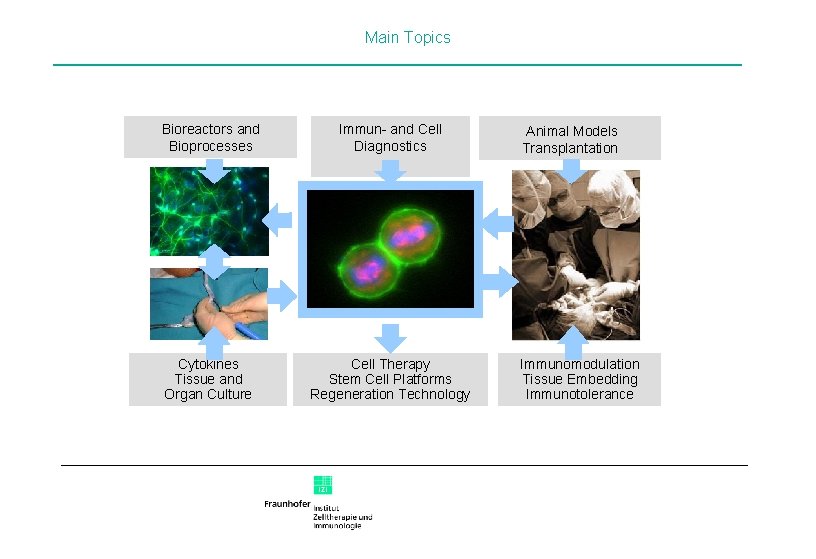
Main Topics Bioreactors and Bioprocesses Immun- and Cell Diagnostics Animal Models Transplantation Zelle Abb. 1 Cytokines Tissue and Organ Culture Cell Therapy Stem Cell Platforms Regeneration Technology Immunomodulation Tissue Embedding Immunotolerance

Research: Immunology GLP GMP GCP 1. Research, development and pre-clinical evaluation (GLP) 2. Integrated R&D solutions supported by the existing technological infrastructure Contract research from concept to proof-of-principle 3. Development of therapeutic agents 4. Biosensor and biochip technologies 5. Preclinical evaluation of therapeutics for human and animal use 6. Full compliance with EU and FDA guidelines including good laboratory practice (GLP) 7. Development of diagnostic systems for pharmaceutical applications

Service GLP GMP GCP 1. Biopharmaceutical manufacturing (GMP) 2. Custom manufacturing of biologics under GMP conditions for direct use in clinical trials 3. GMP-manufacturing of cell-based therapeutics, therapeutic recombinant proteins and antibodies 4. advice for buildup GMP-conform processes 5. Renting of clean room capacity including consent for customer-owned manufacturing permit 6. Practical support for GMP-compliant setup of manufacturing processes 7. Contract manufacturing

Service GLP GMP GCP Implementing novel therapies into the clinic (GCP) Close relationship with Leipzig University hospital permits access to walk-in-clinic infrastructure. In-house laboratories are similarly available for immunodiagnostic use. § § § Close cooperation with University clinic allows product testing in clinical trials (Phase I – II) Quality Management and GCP conform procedures Continuous service from trial design, through manufacture and approval

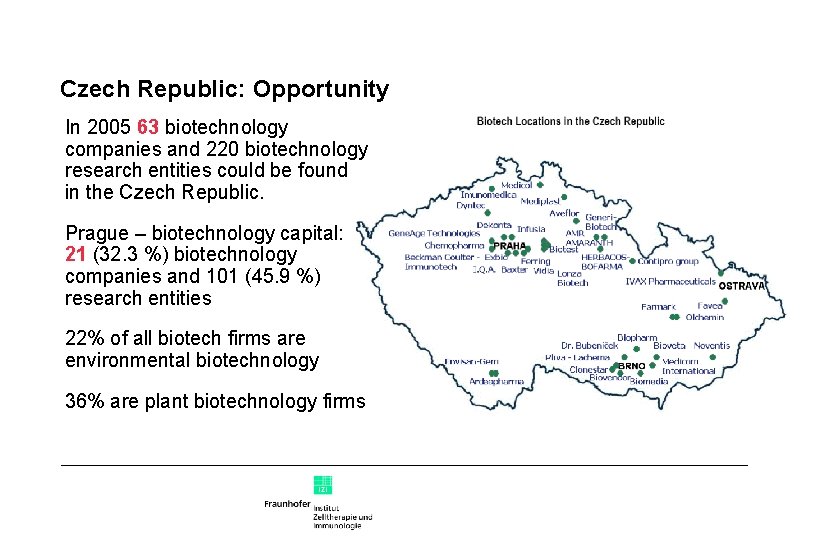
Czech Republic: Opportunity In 2005 63 biotechnology companies and 220 biotechnology research entities could be found in the Czech Republic. Prague – biotechnology capital: 21 (32. 3 %) biotechnology companies and 101 (45. 9 %) research entities 22% of all biotech firms are environmental biotechnology 36% are plant biotechnology firms

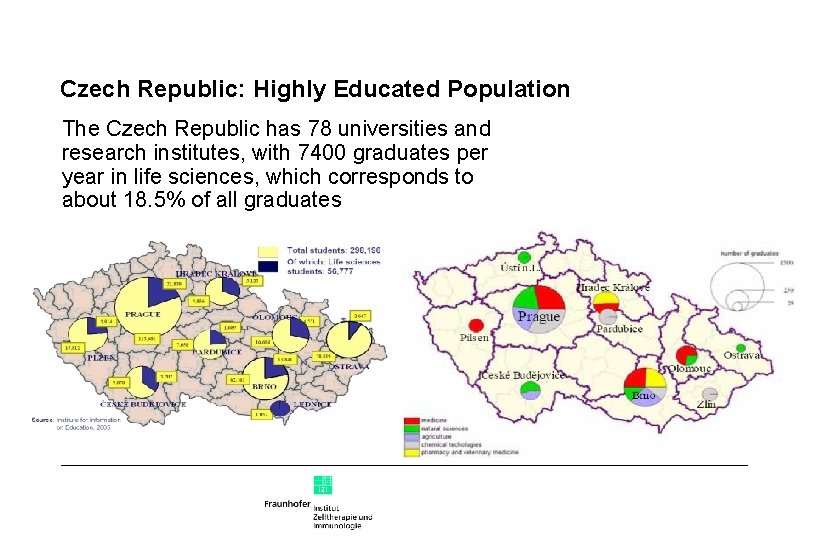
Czech Republic: Highly Educated Population The Czech Republic has 78 universities and research institutes, with 7400 graduates per year in life sciences, which corresponds to about 18. 5% of all graduates

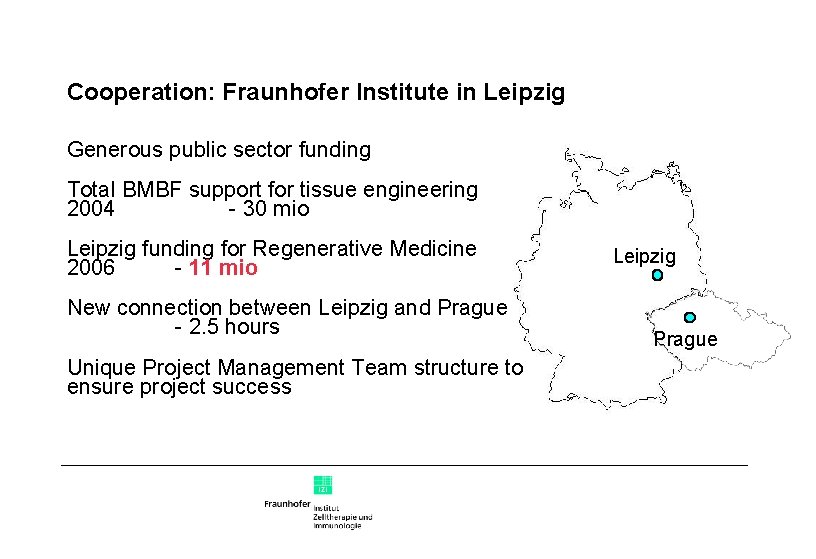
Cooperation: Fraunhofer Institute in Leipzig Generous public sector funding Total BMBF support for tissue engineering 2004 - 30 mio Leipzig funding for Regenerative Medicine 2006 - 11 mio New connection between Leipzig and Prague - 2. 5 hours Unique Project Management Team structure to ensure project success Leipzig Prague

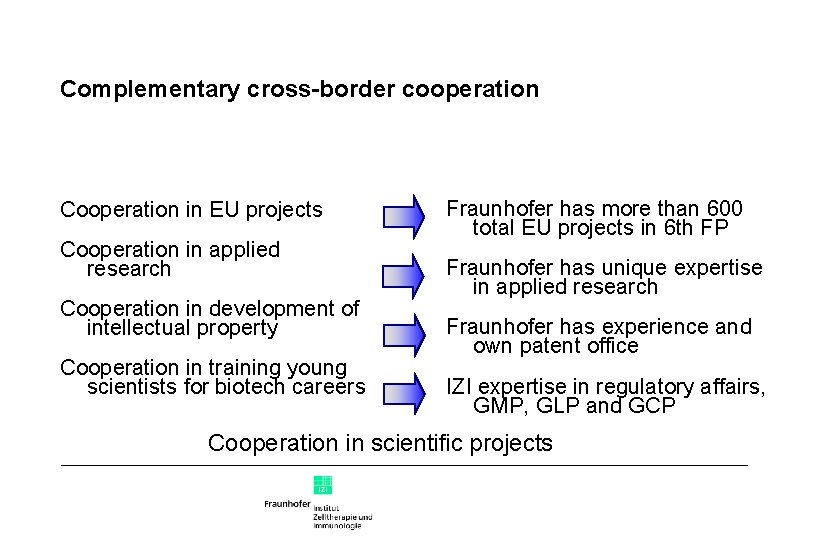
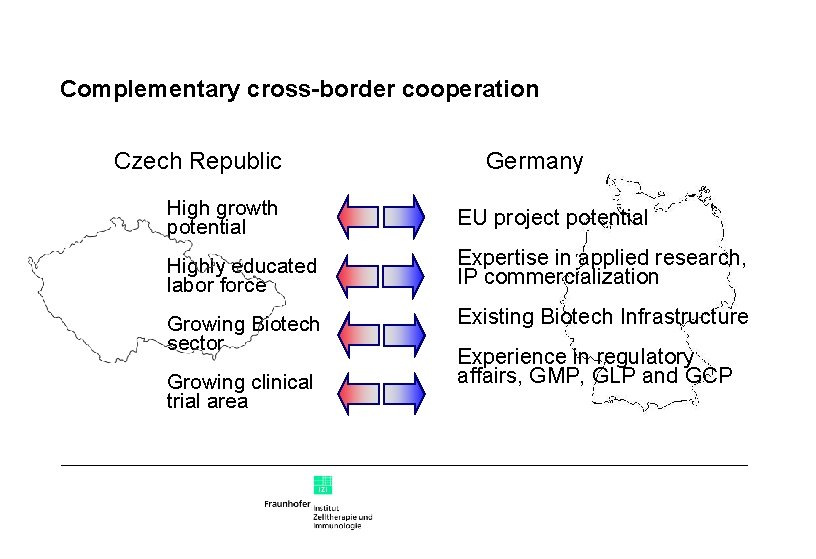
Complementary cross-border cooperation Cooperation in EU projects Cooperation in applied research Cooperation in development of intellectual property Cooperation in training young scientists for biotech careers Fraunhofer has more than 600 total EU projects in 6 th FP Fraunhofer has unique expertise in applied research Fraunhofer has experience and own patent office IZI expertise in regulatory affairs, GMP, GLP and GCP Cooperation in scientific projects

Complementary cross-border cooperation Czech Republic Germany High growth potential EU project potential Highly educated labor force Expertise in applied research, IP commercialization Growing Biotech sector Existing Biotech Infrastructure Growing clinical trial area Experience in regulatory affairs, GMP, GLP and GCP

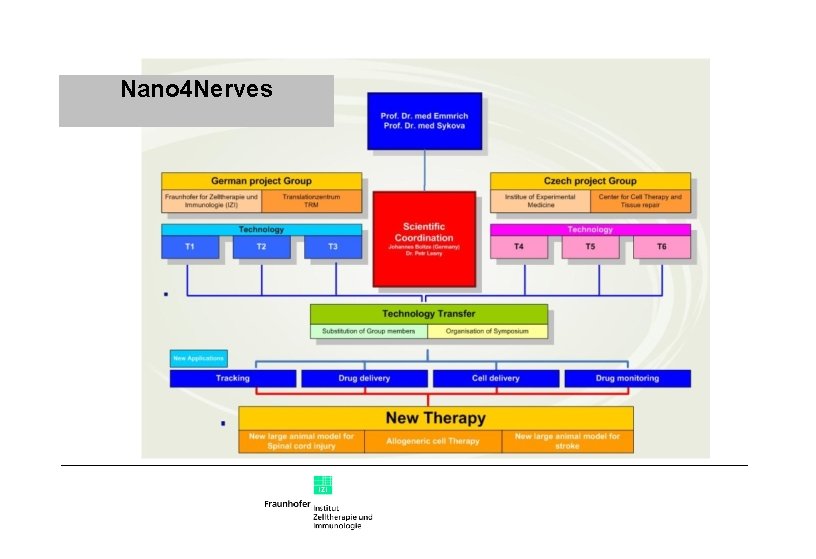
Nano 4 Nerves

Forschung: Service Project Service Team Head: Dr. Wilhelm Gerdes Business Development Public Relations Investment and Grant Funding Patent- & Licence consultation Project Management Strategic Planning

Vielen Dank für Ihre Aufmerksamkeit!
 Fraunhofer institute for cell therapy and immunology
Fraunhofer institute for cell therapy and immunology Fraunhofer
Fraunhofer Fraunhofer
Fraunhofer Fraunhofer
Fraunhofer Fresnel and fraunhofer diffraction
Fresnel and fraunhofer diffraction Diffraction by circular aperature
Diffraction by circular aperature Fraunhöfer
Fraunhöfer Fresnel and fraunhofer diffraction difference
Fresnel and fraunhofer diffraction difference Diffraction
Diffraction L
L Fraunhofer
Fraunhofer Fraunhofer diffraction pattern circular aperture
Fraunhofer diffraction pattern circular aperture Fraunhofer
Fraunhofer Harald egner
Harald egner Fraunhofer
Fraunhofer Fraunhofer
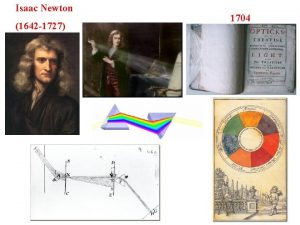
Fraunhofer Isaac orbitals
Isaac orbitals Fraunhofer
Fraunhofer