The Four Organic Compounds Essential Questions What is




- Slides: 13

The Four Organic Compounds Essential Questions: What is “Organic? ” What are the 4 major Organic Compounds? How are they made? What are they used for?

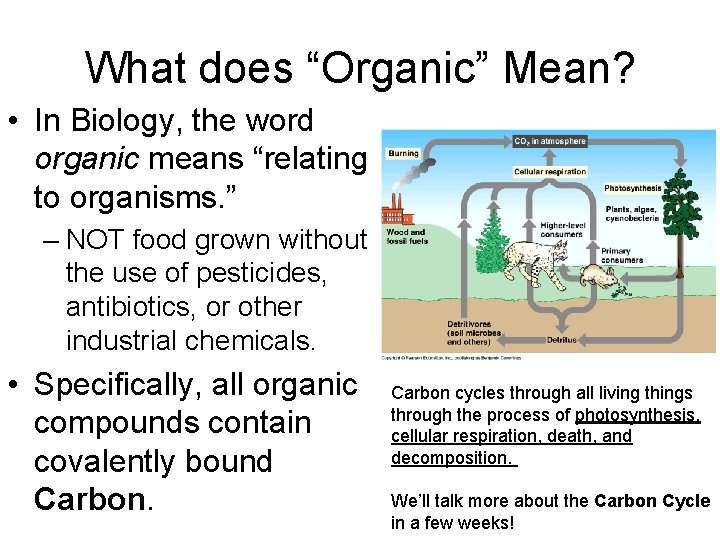
What does “Organic” Mean? • In Biology, the word organic means “relating to organisms. ” – NOT food grown without the use of pesticides, antibiotics, or other industrial chemicals. • Specifically, all organic compounds contain covalently bound Carbon cycles through all living things through the process of photosynthesis, cellular respiration, death, and decomposition. We’ll talk more about the Carbon Cycle in a few weeks!

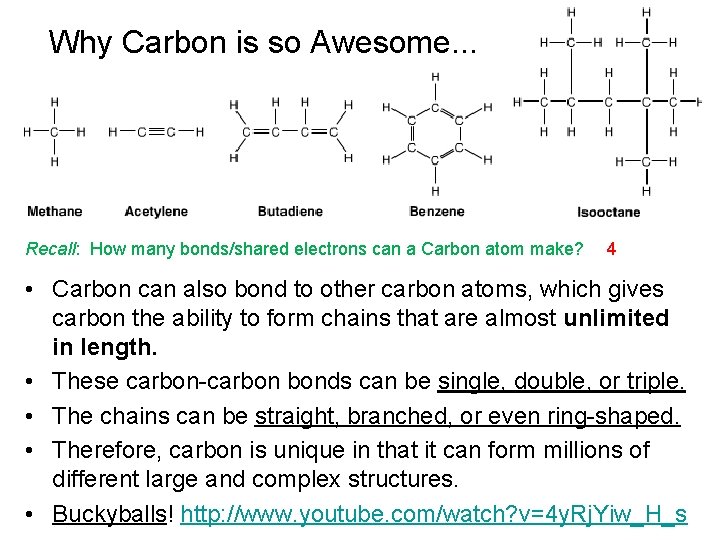
Why Carbon is so Awesome. . . Recall: How many bonds/shared electrons can a Carbon atom make? 4 • Carbon can also bond to other carbon atoms, which gives carbon the ability to form chains that are almost unlimited in length. • These carbon-carbon bonds can be single, double, or triple. • The chains can be straight, branched, or even ring-shaped. • Therefore, carbon is unique in that it can form millions of different large and complex structures. • Buckyballs! http: //www. youtube. com/watch? v=4 y. Rj. Yiw_H_s

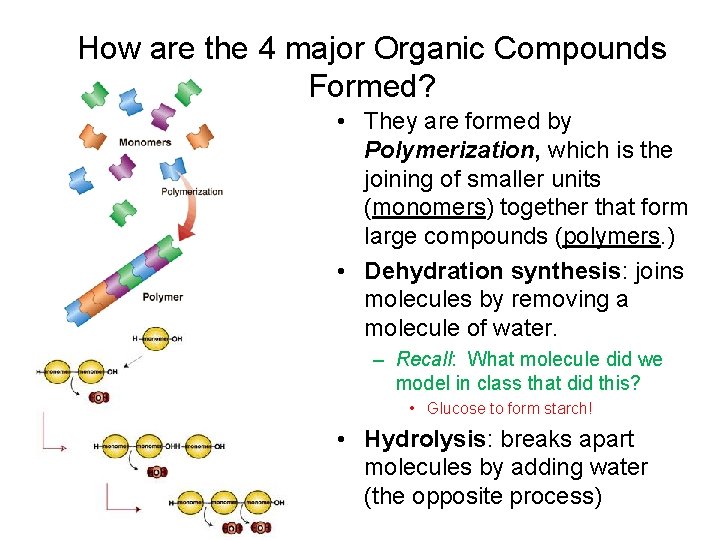
How are the 4 major Organic Compounds Formed? • They are formed by Polymerization, which is the joining of smaller units (monomers) together that form large compounds (polymers. ) • Dehydration synthesis: joins molecules by removing a molecule of water. – Recall: What molecule did we model in class that did this? • Glucose to form starch! • Hydrolysis: breaks apart molecules by adding water (the opposite process)

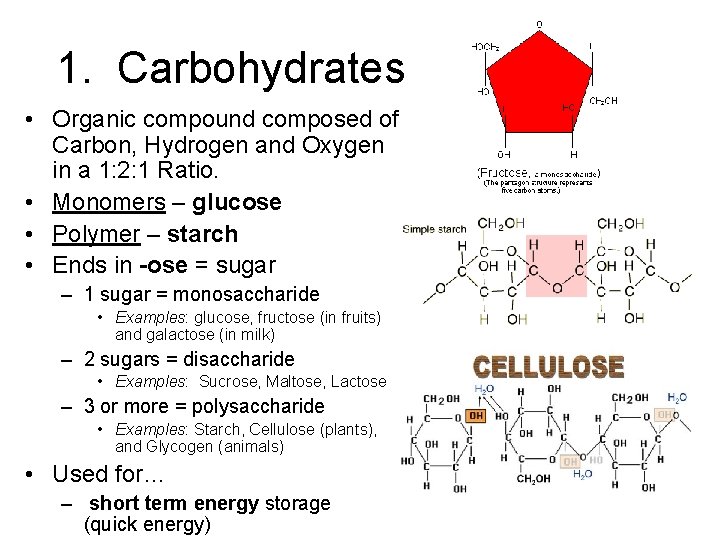
1. Carbohydrates • Organic compound composed of Carbon, Hydrogen and Oxygen in a 1: 2: 1 Ratio. • Monomers – glucose • Polymer – starch • Ends in -ose = sugar – 1 sugar = monosaccharide • Examples: glucose, fructose (in fruits) and galactose (in milk) – 2 sugars = disaccharide • Examples: Sucrose, Maltose, Lactose – 3 or more = polysaccharide • Examples: Starch, Cellulose (plants), and Glycogen (animals) • Used for… – short term energy storage (quick energy)

Why Carbohydrates? • Many animals store extra sugar as glycogen. – Glycogen stored in your muscles supplies energy for movement. – Glycogen stored in your liver is released when the glucose (sugar) in your blood runs low. • Recall: This maintaining a balance in the body is called what? – Homeostasis! • Plants store excess sugar as starch. • Plants also make cellulose, a strong, rigid fiber used for support.

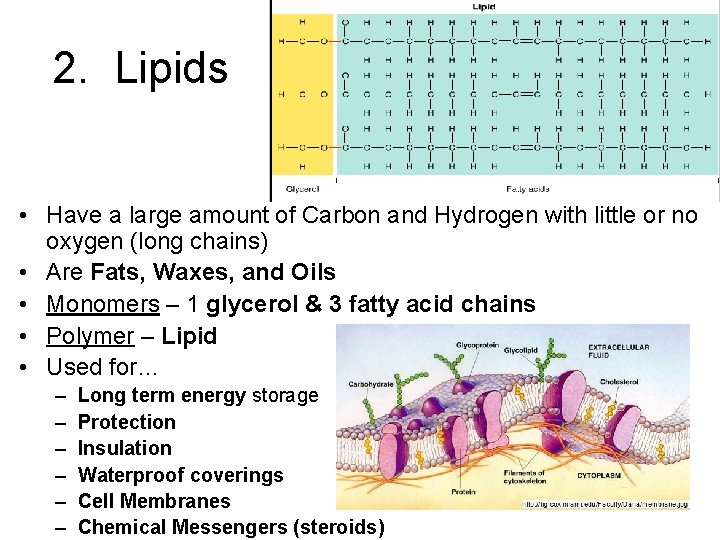
2. Lipids • Have a large amount of Carbon and Hydrogen with little or no oxygen (long chains) • Are Fats, Waxes, and Oils • Monomers – 1 glycerol & 3 fatty acid chains • Polymer – Lipid • Used for… – – – Long term energy storage Protection Insulation Waterproof coverings Cell Membranes Chemical Messengers (steroids)

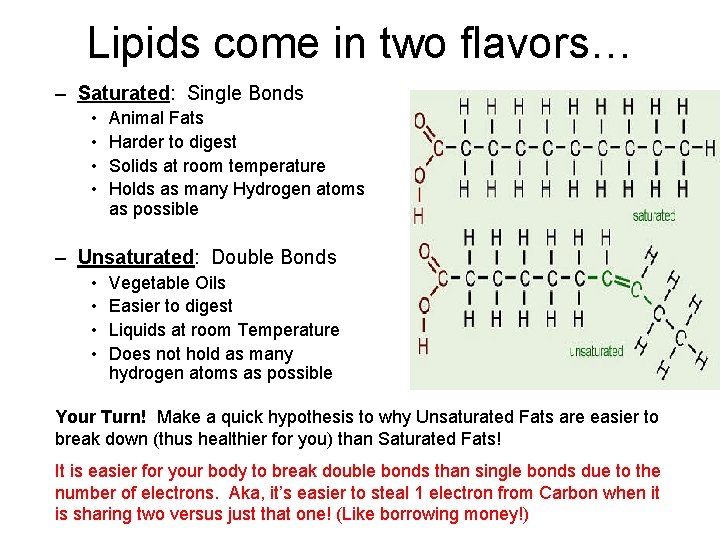
Lipids come in two flavors… – Saturated: Single Bonds • • Animal Fats Harder to digest Solids at room temperature Holds as many Hydrogen atoms as possible – Unsaturated: Double Bonds • • Vegetable Oils Easier to digest Liquids at room Temperature Does not hold as many hydrogen atoms as possible Your Turn! Make a quick hypothesis to why Unsaturated Fats are easier to break down (thus healthier for you) than Saturated Fats! It is easier for your body to break double bonds than single bonds due to the number of electrons. Aka, it’s easier to steal 1 electron from Carbon when it is sharing two versus just that one! (Like borrowing money!)

Common Misconceptions: Lipids – Good & Bad Cholesterol Real Questions: http: //www. youtube. com/watch? v=URDj. XV 9 s 4 c. Y You’ll be amazed at what you find online about the different types of cholesterol! You should check it out!

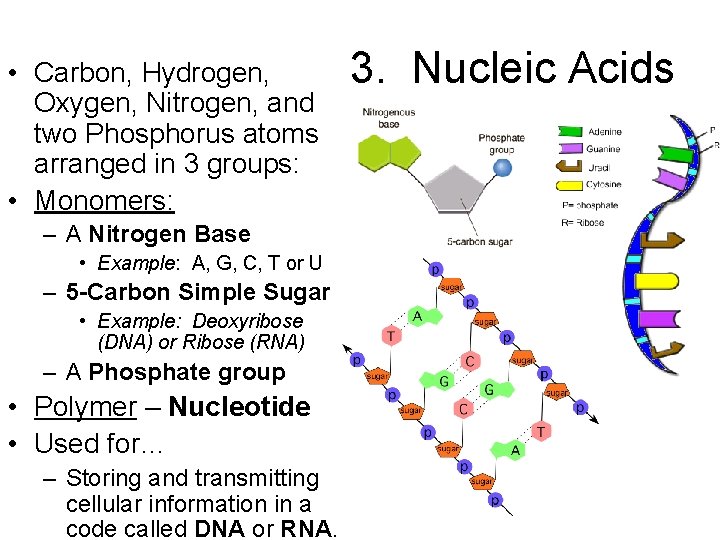
• Carbon, Hydrogen, Oxygen, Nitrogen, and two Phosphorus atoms arranged in 3 groups: • Monomers: – A Nitrogen Base • Example: A, G, C, T or U – 5 -Carbon Simple Sugar • Example: Deoxyribose (DNA) or Ribose (RNA) – A Phosphate group • Polymer – Nucleotide • Used for… – Storing and transmitting cellular information in a code called DNA or RNA. 3. Nucleic Acids

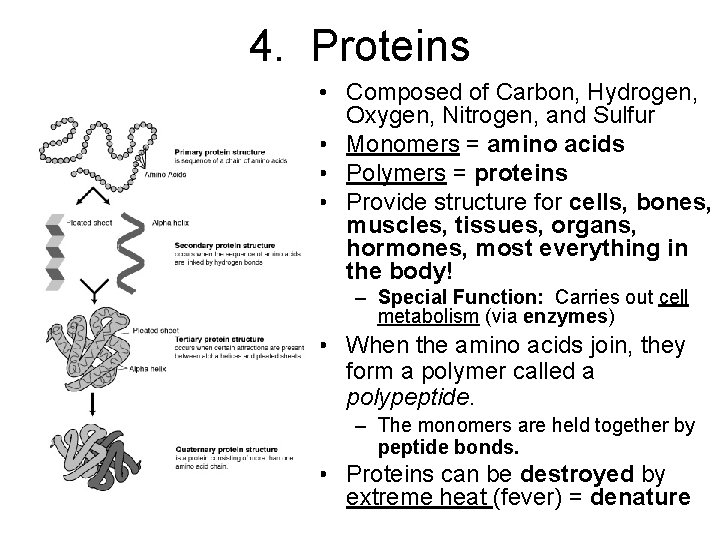
4. Proteins • Composed of Carbon, Hydrogen, Oxygen, Nitrogen, and Sulfur • Monomers = amino acids • Polymers = proteins • Provide structure for cells, bones, muscles, tissues, organs, hormones, most everything in the body! – Special Function: Carries out cell metabolism (via enzymes) • When the amino acids join, they form a polymer called a polypeptide. – The monomers are held together by peptide bonds. • Proteins can be destroyed by extreme heat (fever) = denature

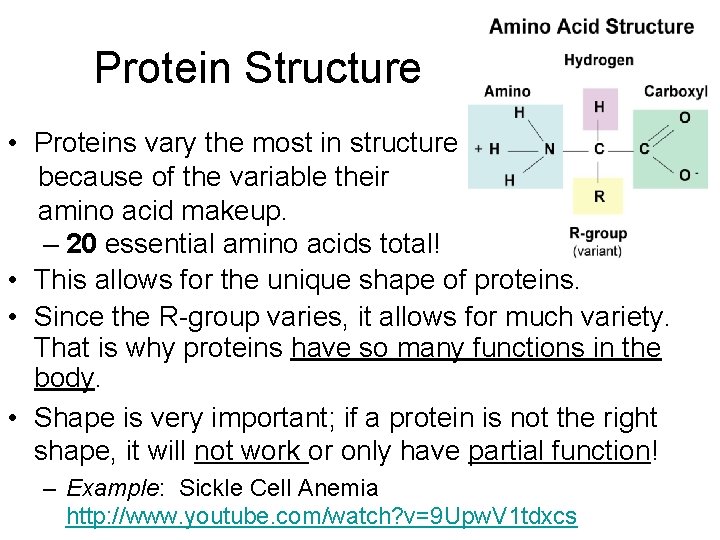
Protein Structure • Proteins vary the most in structure because of the variable their amino acid makeup. – 20 essential amino acids total! • This allows for the unique shape of proteins. • Since the R-group varies, it allows for much variety. That is why proteins have so many functions in the body. • Shape is very important; if a protein is not the right shape, it will not work or only have partial function! – Example: Sickle Cell Anemia http: //www. youtube. com/watch? v=9 Upw. V 1 tdxcs

Safety for today’s lab: Wear goggles at Sugar station Wear gloves at sugar and Protein stations